.A sample of alfalfa meal weighing 2.0 g is analyzed by Kjeldahl method for the percentage of nitrogen. The liberated NH3 is caught in a solution of H3B03, and 8.23 ml of HCI are required in the subsequent titration. A sample of pure (NH4)2SO4 (132.12) weighing 0.61 g is treated with excess NaOH and the liberated NH3 (17.04) is also caught in H3BO3. The resulting solution requires 20 ml of the acid for titration. Calculate the percentage of protein in the sample using 6.25 as the factor.
.A sample of alfalfa meal weighing 2.0 g is analyzed by Kjeldahl method for the percentage of nitrogen. The liberated NH3 is caught in a solution of H3B03, and 8.23 ml of HCI are required in the subsequent titration. A sample of pure (NH4)2SO4 (132.12) weighing 0.61 g is treated with excess NaOH and the liberated NH3 (17.04) is also caught in H3BO3. The resulting solution requires 20 ml of the acid for titration. Calculate the percentage of protein in the sample using 6.25 as the factor.
Chapter20: Applications Of Oxidation/reduction Titrations
Section: Chapter Questions
Problem 20.25QAP
Related questions
Question
3
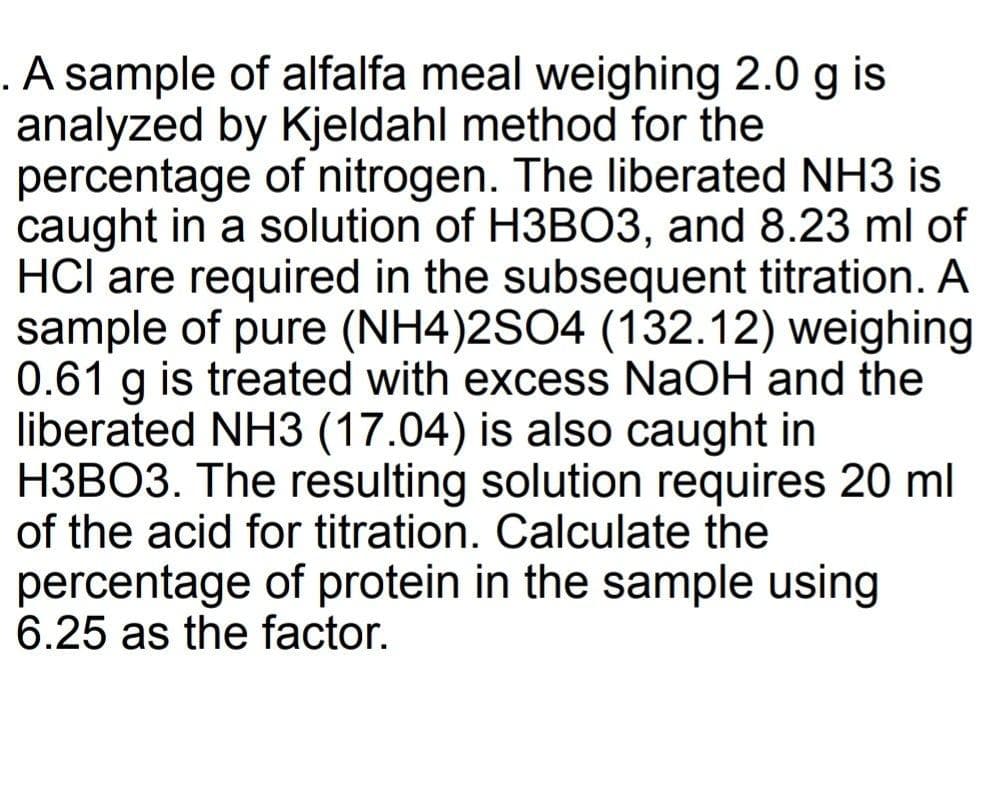
Transcribed Image Text:.A sample of alfalfa meal weighing 2.0 g is
analyzed by Kjeldahl method for the
percentage of nitrogen. The liberated NH3 is
caught in a solution of H3BO3, and 8.23 ml of
HCl are required in the subsequent titration. A
sample of pure (NH4)2SO4 (132.12) weighing
0.61 g is treated with excess NaOH and the
liberated NH3 (17.04) is also caught in
H3BO3. The resulting solution requires 20 ml
of the acid for titration. Calculate the
percentage of protein in the sample using
6.25 as the factor.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning