.What is the type of reaction involved? SA + SB SB + SA WA + SB WB + SA 2.What is the preferred indicator for the titration? Methyl red Methyl yellow Methyl orange Phenolphthalein 3.What is the pka of HPr? Present your answer to the 3ʳᵈ decimal place for this item. Your answer here is to be used for the next item/s.
.What is the type of reaction involved? SA + SB SB + SA WA + SB WB + SA 2.What is the preferred indicator for the titration? Methyl red Methyl yellow Methyl orange Phenolphthalein 3.What is the pka of HPr? Present your answer to the 3ʳᵈ decimal place for this item. Your answer here is to be used for the next item/s.
Chapter14: Principles Of Neutralization Titrations
Section: Chapter Questions
Problem 14.6QAP
Related questions
Question
1.What is the type of reaction involved?
SA + SB
SB + SA
WA + SB
WB + SA
2.What is the preferred indicator for the titration?
Methyl red
Methyl yellow
Methyl orange
Phenolphthalein
3.What is the pka of HPr?
Present your answer to the 3ʳᵈ decimal place for this item. Your answer here is to be used for the next item/s.
4.What will be the change in pH after adding 13.4ml of the titrant? *
Present your answer to the 2ⁿᵈ decimal place for convenience in graphing.
5.What will be the change in pH after adding 26 ml of the titrant?
Present your answer to the 2ⁿᵈ decimal place
6.What is the total volume at equivalence point?
Present your answer to the 2ⁿᵈ decimal place
7.What is the pH at equivalence point?
Present your answer to the 2ⁿᵈ decimal place
8.Upload a clear and well-lit image of a hand-written, completely plotted and labeled titration curve .
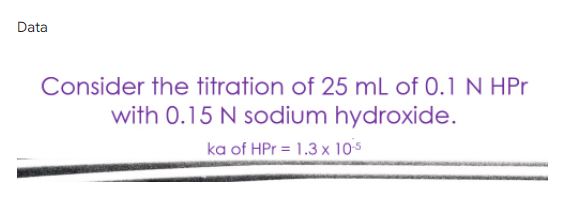
Transcribed Image Text:Data
Consider the titration of 25 mL of 0.1 N HPr
with 0.15 N sodium hydroxide.
ka of HPr = 1.3 × 10s
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning



Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning