1 2 3 4 5 6 7 8 9 89 check on the appropriate box. The first five substances were done for you. TYPES OF CRYSTAL 19 20 Substance Kr HF NO Al K₂S NH3 HC1 KMnO4 10 11 12 13 14 15 16 17 18 PH 1₂ Cu SO₂ CO₂ Si SiO₂ Cgraphite CH3OH Ag Ba(OH)2 CaO Dispersion forces Molecular Dipole- dipole attraction Hydrogen bonds Metallic Ionic Metallic bonds bonds Covalent Network Ionic Covalent bonds
1 2 3 4 5 6 7 8 9 89 check on the appropriate box. The first five substances were done for you. TYPES OF CRYSTAL 19 20 Substance Kr HF NO Al K₂S NH3 HC1 KMnO4 10 11 12 13 14 15 16 17 18 PH 1₂ Cu SO₂ CO₂ Si SiO₂ Cgraphite CH3OH Ag Ba(OH)2 CaO Dispersion forces Molecular Dipole- dipole attraction Hydrogen bonds Metallic Ionic Metallic bonds bonds Covalent Network Ionic Covalent bonds
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter21: Structure And Bonding In Solids
Section: Chapter Questions
Problem 31P
Related questions
Question
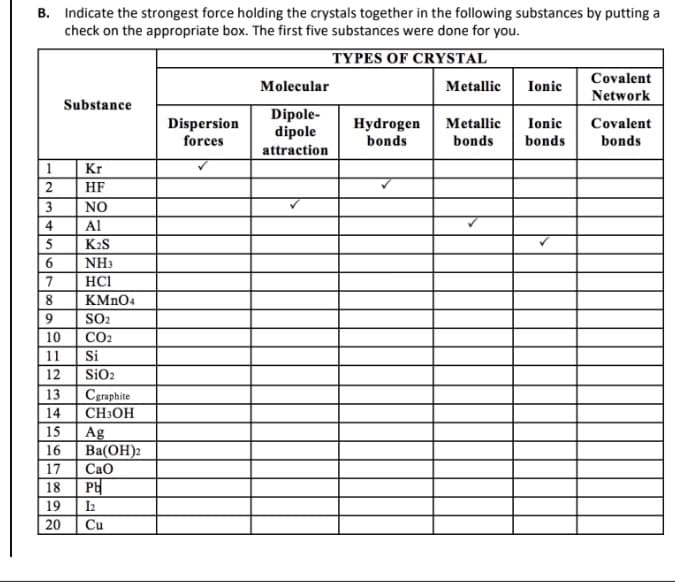
Transcribed Image Text:B. Indicate the strongest force holding the crystals together in the following substances by putting a
check on the appropriate box. The first five substances were done for you.
TYPES OF CRYSTAL
1
2
3
4
5
6
7
8
9
Substance
Kr
HF
NO
Al
K₂S
NH3
HC1
KMnO4
SO₂
CO₂
Si
SiO₂
10
11
12
13
14
15 Ag
16
17
18
19 12
20
Cgraphite
CH3OH
Ba(OH)2
CaO
PH
Cu
Dispersion
forces
Molecular
Dipole-
dipole
attraction
Hydrogen
bonds
Metallic Ionic
Metallic
bonds
Ionic
bonds
Covalent
Network
Covalent
bonds
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning