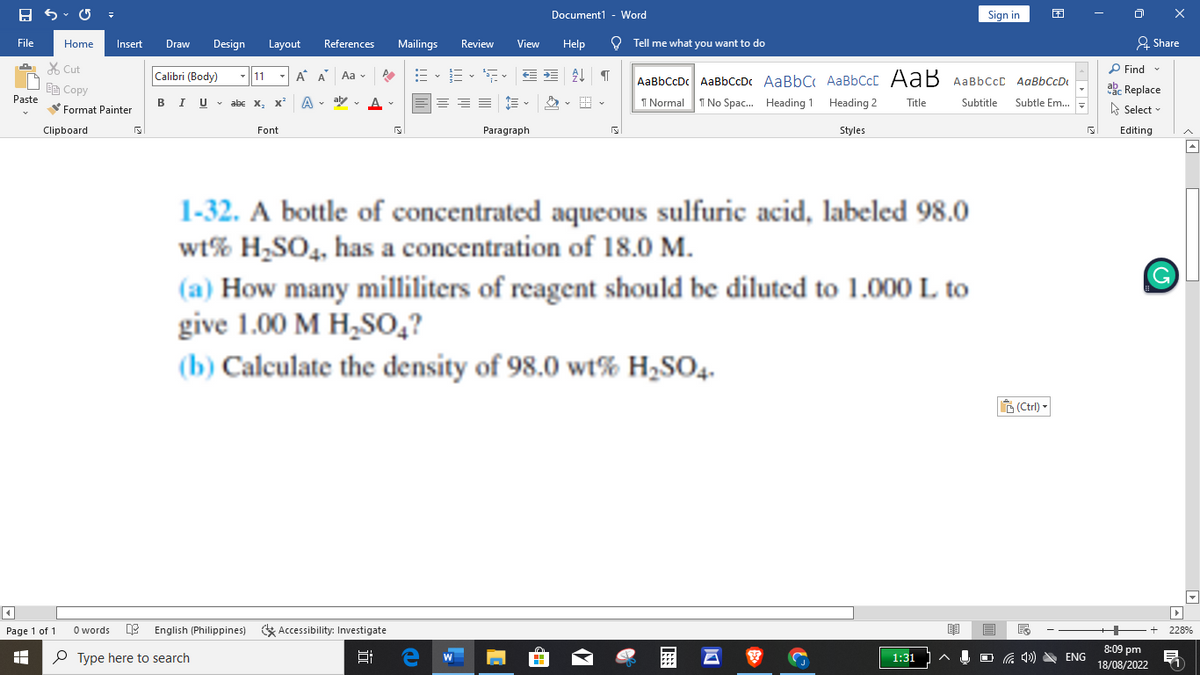
1-32. A bottle of concentrated aqueous sulfuric acid, labeled 98.0 wt% H₂SO4, has a concentration of 18.0 M. (a) How many milliliters of reagent should be diluted to 1.000 L to give 1.00 M H₂SO4? (b) Calculate the density of 98.0 wt% H₂SO4.
1-32. A bottle of concentrated aqueous sulfuric acid, labeled 98.0 wt% H₂SO4, has a concentration of 18.0 M. (a) How many milliliters of reagent should be diluted to 1.000 L to give 1.00 M H₂SO4? (b) Calculate the density of 98.0 wt% H₂SO4.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 80AP
Related questions
Question
Transcribed Image Text:B
File
Paste
E
+
Home
Cut
E Copy
4
Page 1 of 1
Clipboard
Format Painter
Insert
0 words
S
Draw Design Layout References
Calibri (Body)
BIU U abe
11 À A Aa
A A
abe X₂ X²
Font
Type here to search
P
English (Philippines) Accessibility: Investigate
Mailings
10
E•E•S•
Review View Help
HE
e W
Document1 - Word
ۦ
Paragraph
1-32. A bottle of concentrated aqueous sulfuric acid, labeled 98.0
wt% H₂SO4, has a concentration of 18.0 M.
(a) How many milliliters of reagent should be diluted to 1.000 L to
give 1.00 M H₂SO4?
(b) Calculate the density of 98.0 wt% H₂SO4-
S
■
Tell me what you want to do
AaBbCcDc AaBbCcDc AaBbC AaBbCct AaB AaBbcct AaBbCcD
Normal 1 No Spac... Heading 1
Subtitle Subtle Em...
Heading 2 Title
Styles
Sign in
1:31
(Ctrl) -
ENG
S
IX
Share
Find
ab Replace
Select
Editing
8:09 pm
18/08/2022
▸►
+ 228%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning



Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
