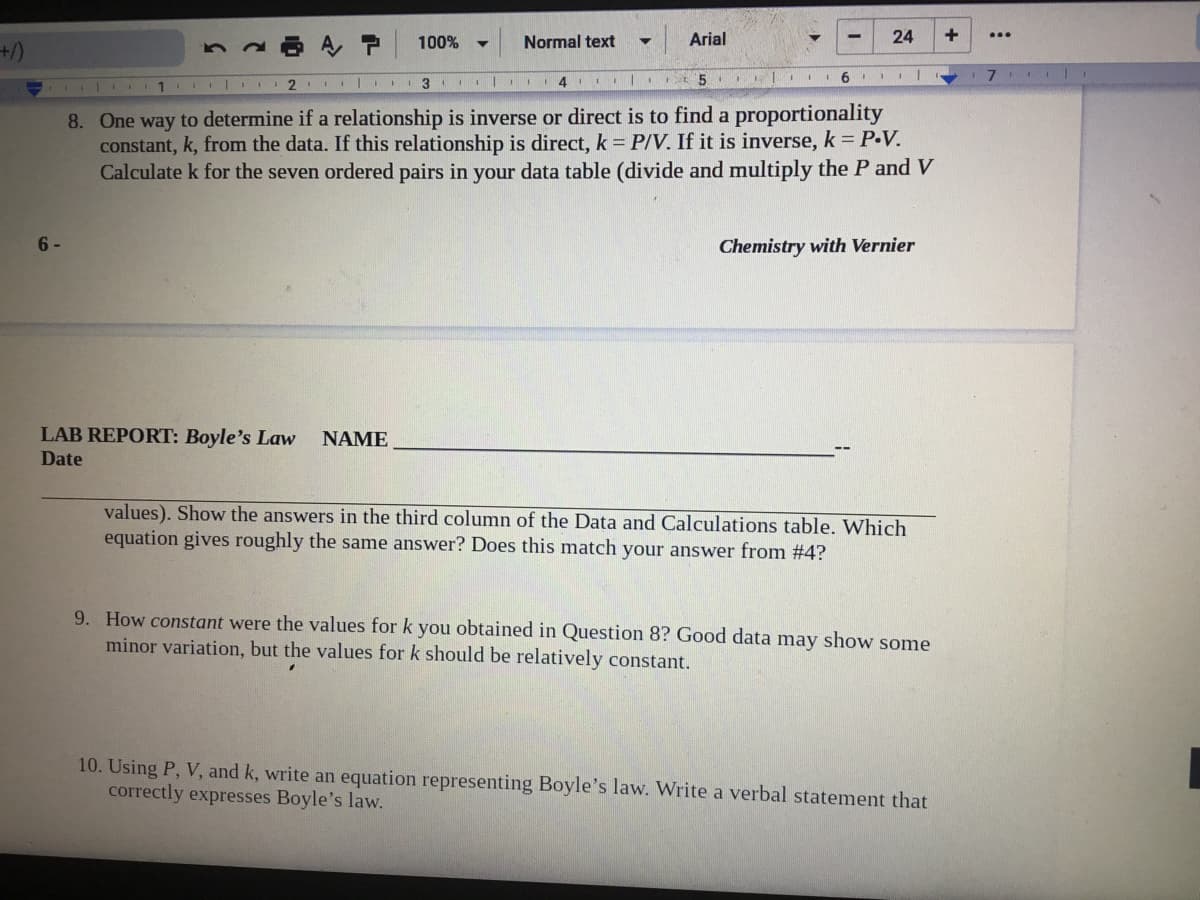
1 8. One way to determine if a relationship is inverse or direct is to find a proportionality constant, k, from the data. If this relationship is direct, k P/V. If it is inverse, k = P-V. Calculate k for the seven ordered pairs in your data table (divide and multiply the P and V
1 8. One way to determine if a relationship is inverse or direct is to find a proportionality constant, k, from the data. If this relationship is direct, k P/V. If it is inverse, k = P-V. Calculate k for the seven ordered pairs in your data table (divide and multiply the P and V
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
Transcribed Image Text:Arial
24
...
100%
Normal text
(/+
| 1 I 2 l 3 I 4 III|
5.
8. One way to determine if a relationship is inverse or direct is to find a proportionality
constant, k, from the data. If this relationship is direct, k P/V. If it is inverse, k = P-V.
Calculate k for the seven ordered pairs in your data table (divide and multiply the P and V
6 -
Chemistry with Vernier
LAB REPORT: Boyle's Law
NAME
Date
values). Show the answers in the third column of the Data and Calculations table. Which
equation gives roughly the same answer? Does this match your answer from #4?
9. How constant were the values for k you obtained in Question 8? Good data may show some
minor variation, but the values for k should be relatively constant.
10. Using P, V, and k, write an equation representing Boyle's law. Write a verbal statement that
correctly expresses Boyle's law.
Transcribed Image Text:100%
nus (Alt+/)
I 21 1
1
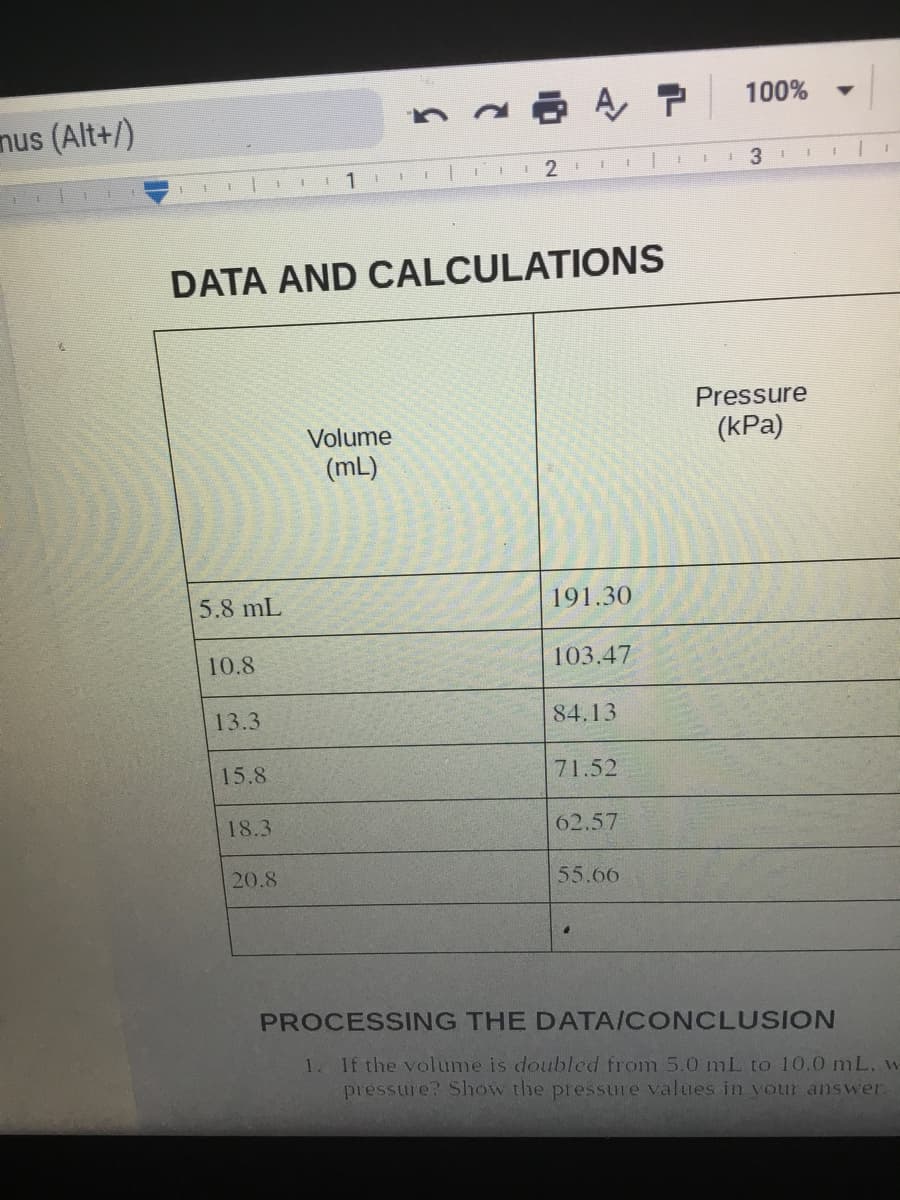
DATA AND CALCULATIONS
Pressure
Volume
(kPa)
(mL)
5.8 mL
191.30
10.8
103.47
13.3
84.13
15.8
71.52
18.3
62.57
20.8
55.66
PROCESSING THE DATA/CONCLUSION
1. If the volume is doulbled from 5.0 mL to 10.0 mL, w
pressure? Slhow the pressure values in your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
