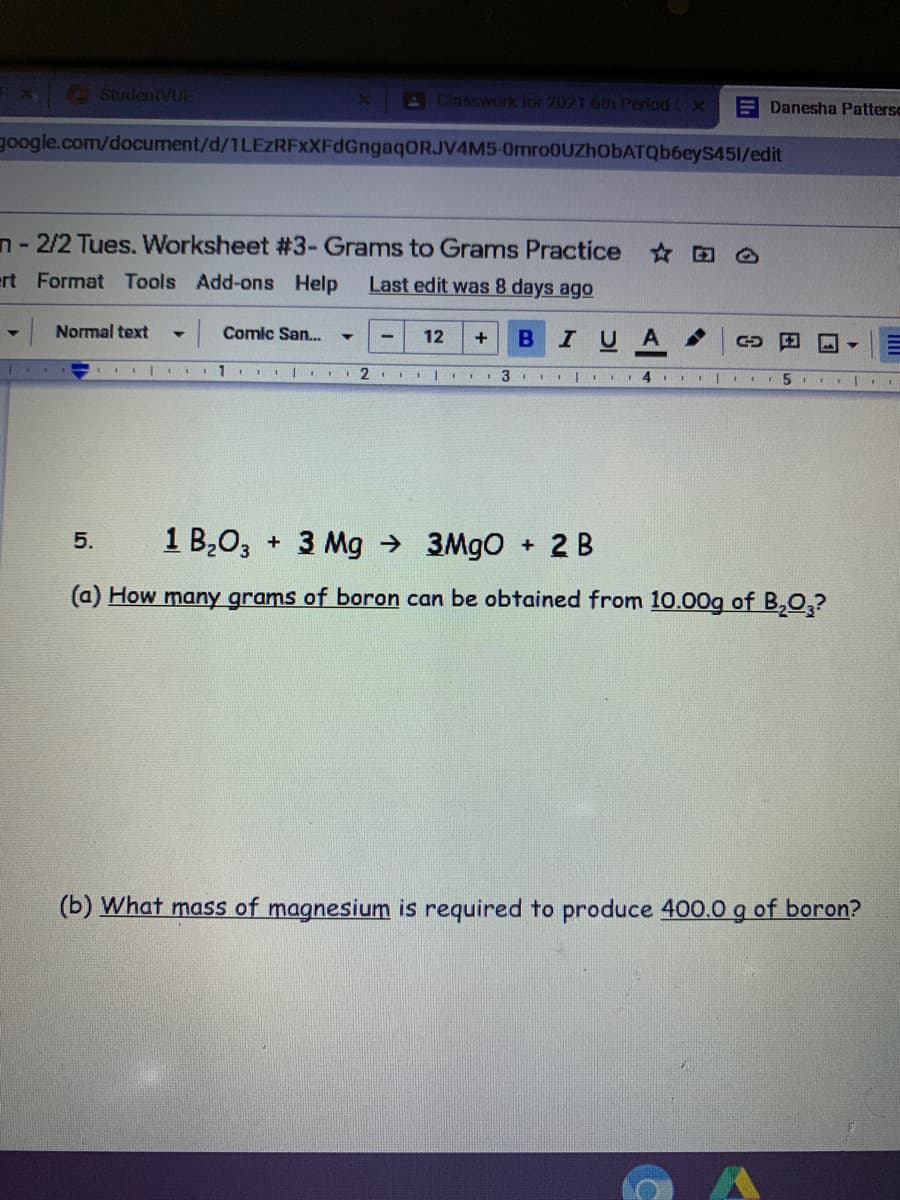
1 B,0, + 3 Mg → 3M9O + 2 B 5. (a) How many grams of boron can be obtained from 10.00g of B,0,? (b) What mass of magnesium is required to produce 400.0 g of boron?
1 B,0, + 3 Mg → 3M9O + 2 B 5. (a) How many grams of boron can be obtained from 10.00g of B,0,? (b) What mass of magnesium is required to produce 400.0 g of boron?
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.30QAP
Related questions
Question
Transcribed Image Text:StudentVUE
AClassworik Tor 2021 Gth Period
E Danesha Pattersc
google.com/document/d/1LEzRFXXFdGngaqORJV4M5-0mro0UZhObATQb6eyS451/edit
n - 2/2 Tues. Worksheet #3- Grams to Grams Practice
ert Format Tools Add-ons Help
Last edit was 8 days ago
Normal text
|Comic San..
12
B
IUA ,
1. |. 2 1 |
3
1 B,0, + 3 Mg →
+ 2 B
5.
3MGO
(a) How many grams of boron can be obtained from 10.00g of B,Q,?
(b) What mass of magnesium is required to produce 400.0 g of boron?
Expert Solution
Step 1
Given balanced equation is as follows.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning