(1 points) Suppose the above solution is cooled to 0 °C. How many grams of A would precipitate from the solution? What is the % recovery of A? % recovery=. mass "recovered" material (purified compound) mass of starting mixture (impure compound) 100
(1 points) Suppose the above solution is cooled to 0 °C. How many grams of A would precipitate from the solution? What is the % recovery of A? % recovery=. mass "recovered" material (purified compound) mass of starting mixture (impure compound) 100
Chapter80: Crystallization: Purification Of Solids
Section: Chapter Questions
Problem 1P
Related questions
Question
I am only confused on part D of this problem. I understand that the 10g of A will dissolve completely and begin to precipitate at approx. 55 degrees celsius, but do not understand how to find the grams precipitated.
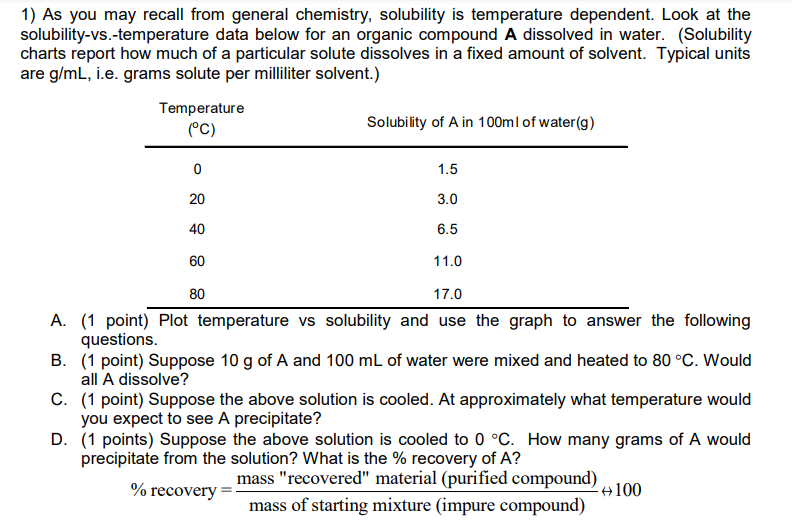
Transcribed Image Text:1) As you may recall from general chemistry, solubility is temperature dependent. Look at the
solubility-vs.-temperature data below for an organic compound A dissolved in water. (Solubility
charts report how much of a particular solute dissolves in a fixed amount of solvent. Typical units
are g/mL, i.e. grams solute per milliliter solvent.)
Temperature
(°C)
0
20
40
60
Solubility of A in 100ml of water (g)
1.5
3.0
6.5
11.0
80
17.0
A. (1 point) Plot temperature vs solubility and use the graph to answer the following
questions.
B. (1 point) Suppose 10 g of A and 100 mL of water were mixed and heated to 80 °C. Would
all A dissolve?
C. (1 point) Suppose the above solution is cooled. At approximately what temperature would
you expect to see A precipitate?
=
D. (1 points) Suppose the above solution is cooled to 0 °C. How many grams of A would
precipitate from the solution? What is the % recovery of A?
% recovery=
mass "recovered" material (purified compound)
mass of starting mixture (impure compound)
-100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning