1) When a mass, m, of cyclohexane is burnt in a constant volume bomb calorimeter with heat capacity Ceal the temperature of the water surrounding the calorimeter is raised by AT- The heat of combustion of the nichrome wire used to ignite the reactive mixture is q. The lengths of the nichrome wire before and after combustion are L, and L, respectively. The combustion reaction is carried out at temerature T and pressure P. The standard molar enthalpy of combustion of cyclohexane is written as AH°. Table 1: Experimental conditions and thermodynamic data for the combustion in the bomb calorimeter A Ho m Ccal q Lo L T P -3951 +/-19 kJ/mol 0.78 +0.002 g 8222.0+/8.0 J/k -9.6 J/cm 9. 39 +0.05 cm 3. 39 +/-0.05 cm 20.65 +/-0.04 C 25.8 +/-1.0 bar
1) When a mass, m, of cyclohexane is burnt in a constant volume bomb calorimeter with heat capacity Ceal the temperature of the water surrounding the calorimeter is raised by AT- The heat of combustion of the nichrome wire used to ignite the reactive mixture is q. The lengths of the nichrome wire before and after combustion are L, and L, respectively. The combustion reaction is carried out at temerature T and pressure P. The standard molar enthalpy of combustion of cyclohexane is written as AH°. Table 1: Experimental conditions and thermodynamic data for the combustion in the bomb calorimeter A Ho m Ccal q Lo L T P -3951 +/-19 kJ/mol 0.78 +0.002 g 8222.0+/8.0 J/k -9.6 J/cm 9. 39 +0.05 cm 3. 39 +/-0.05 cm 20.65 +/-0.04 C 25.8 +/-1.0 bar
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 75AP
Related questions
Question
Given that the magnitudes of the standard molar heat of combustion of graphite and hvdrogen (H2) are -285.83 and -395. 51k]/mol respectively, determine the standard molar head of formation (Deltaa H) of cyclohexane using the appropriate data from Table 1. (Pay attention to the signs!) Need delta H and its standard deviation, unit is kJ/mol
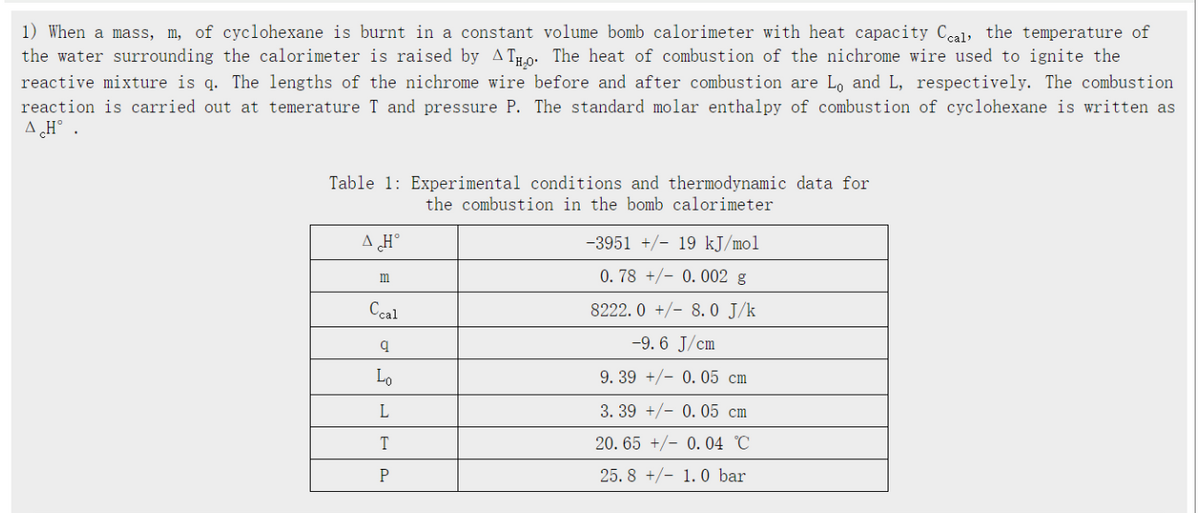
Transcribed Image Text:1) When a mass, m, of cyclohexane is burnt in a constant volume bomb calorimeter with heat capacity Ceal the temperature of
the water surrounding the calorimeter is raised by AT- The heat of combustion of the nichrome wire used to ignite the
reactive mixture is q. The lengths of the nichrome wire before and after combustion are L, and L, respectively. The combustion
reaction is carried out at temerature T and pressure P. The standard molar enthalpy of combustion of cyclohexane is written as
AH°.
Table 1: Experimental conditions and thermodynamic data for
the combustion in the bomb calorimeter
A Ho
m
Ccal
q
Lo
L
T
P
-3951 +/-19 kJ/mol
0.78 +0.002 g
8222.0+/8.0 J/k
-9.6 J/cm
9. 39 +0.05 cm
3. 39 +/-0.05 cm
20.65 +/-0.04 C
25.8 +/-1.0 bar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning