1. A pH change of 0.2 units requires the addition of 0.05 mL of NaOH. You are probably at what point in the titration? a. very close to equivalence point b. after equivalence point c. either before or after the equivalence point d. before equivalence point 2. A pH change of 0.2 units requires the addition of 1.50 mL of NaOH. You are probably at what point in the titration? a. after equivalence point b. either beforeor after the equivalence point c. very close to equivalence point d. before the equivalence point 3. Looking at the graph provided, which of the following indicators could be used to find the equivalence point for this titration the conventional way? Select all that apply. a. methyl orange (turns red to yellow from a pH of 3.1 to 4.4) b. methyl red (turns red to yellow from a pH of 4.2 to 6.3) c. cresol red (turns yellow to red from a pH of 7.2 to 8.8) d. thymol blue (turns yellow to red form a pH of 8.0 to 9.6) e. phenolphthalein (turns colorless to dark pink from a pH of 8.3 to 10.0) f. alizarin yellow R (turns yellow to orange -red from a pH of 10.1 to 12.0) 4. In a potentiometric titration is the titration stopped as soon as the equivalence point is reached? Why or why not? a. No. The 2-4 mL portions of base should be added until a buret is empty. Otherwise the experiment is not completed. b. Yes. There is no need to continue the titration after the equivalence point is reached because only the equivalence point is the essential information in this experiment. c. No. Five or six more portions of 2-4 mL portions of base should be added otherwise the equivalence point cannot be determined accurately from the graph. 5. Does it matter how much water is used to dissolve the unknown monoprotic acid? Explain your answer. a.Yes. Adding a higher (or lower) amount of water would change the number of moles of the unknown monoprotic acid in the solution, so the equivalence point will be reached at different pH. b. No. Adding water does not change the number of moles of the unknown monoprotic acid, so the amount of water is insignificant. c. Yes. Adding a higher (or lower) amount of water would change the initial concentration of unknown monoprotic acid, so the equivalence point will be reached at different pH.
1. A pH change of 0.2 units requires the addition of 0.05 mL of NaOH. You are probably at what point in the titration?
a. very close to equivalence point
b. after equivalence point
c. either before or after the equivalence point
d. before equivalence point
2. A pH change of 0.2 units requires the addition of 1.50 mL of NaOH. You are probably at what point in the titration?
a. after equivalence point
b. either beforeor after the equivalence point
c. very close to equivalence point
d. before the equivalence point
3. Looking at the graph provided, which of the following indicators could be used to find the equivalence point for this titration the conventional way? Select all that apply.
a. methyl orange (turns red to yellow from a pH of 3.1 to 4.4)
b. methyl red (turns red to yellow from a pH of 4.2 to 6.3)
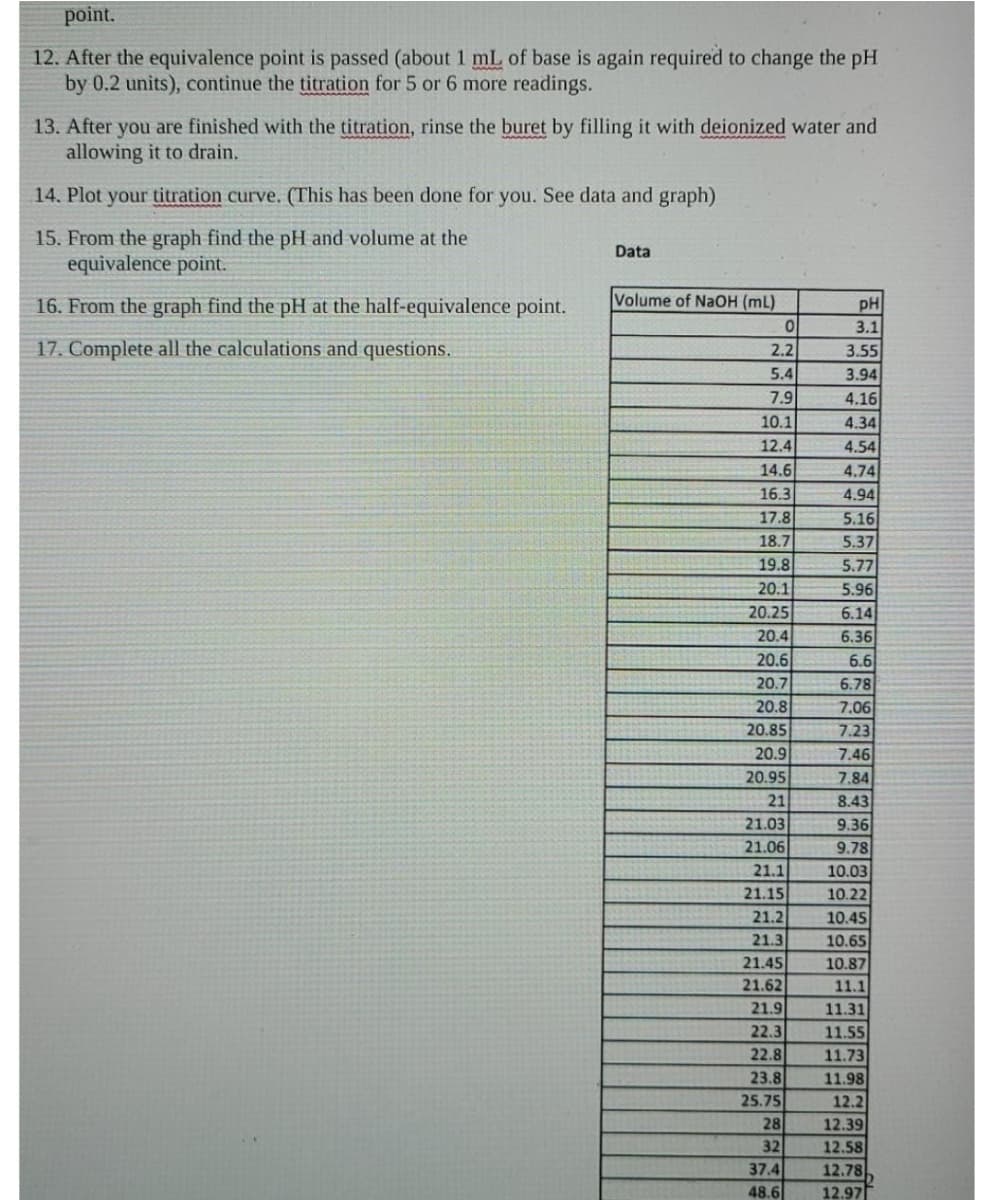
c. cresol red (turns yellow to red from a pH of 7.2 to 8.8)
d. thymol blue (turns yellow to red form a pH of 8.0 to 9.6)
e. phenolphthalein (turns colorless to dark pink from a pH of 8.3 to 10.0)
f. alizarin yellow R (turns yellow to orange -red from a pH of 10.1 to 12.0)
4. In a potentiometric titration is the titration stopped as soon as the equivalence point is reached? Why or why not?
a. No. The 2-4 mL portions of base should be added until a buret is empty. Otherwise the experiment is not completed.
b. Yes. There is no need to continue the titration after the equivalence point is reached because only the equivalence point is the essential information in this experiment.
c. No. Five or six more portions of 2-4 mL portions of base should be added otherwise the equivalence point cannot be determined accurately from the graph.
5. Does it matter how much water is used to dissolve the unknown monoprotic acid? Explain your answer.
a.Yes. Adding a higher (or lower) amount of water would change the number of moles of the unknown monoprotic acid in the solution, so the equivalence point will be reached at different pH.
b. No. Adding water does not change the number of moles of the unknown monoprotic acid, so the amount of water is insignificant.
c. Yes. Adding a higher (or lower) amount of water would change the initial concentration of unknown monoprotic acid, so the equivalence point will be reached at different pH.

Trending now
This is a popular solution!
Step by step
Solved in 2 steps








