1. A solution containing 50% KOH is fed at a rate of 1000 mol/h to an evaporator to produce a concentrated solution containing 80%mol KOH. The concentrated solution is then fed to a crystallizer to produce a filter cake containing 90% pure KOH and 10% mother liquor (60% KOH solution). The rest of the mother liquor (60% KOH solution) is recycled back to the evaporator. Determine the values of B, C, P and W. Refer to the figure below. H,0 TW F-1000 mole/ B, 8O% KOH P, S0% pure KOH filter 50% by mole KOH 10% (s0s KOH solution) 60% KOH
1. A solution containing 50% KOH is fed at a rate of 1000 mol/h to an evaporator to produce a concentrated solution containing 80%mol KOH. The concentrated solution is then fed to a crystallizer to produce a filter cake containing 90% pure KOH and 10% mother liquor (60% KOH solution). The rest of the mother liquor (60% KOH solution) is recycled back to the evaporator. Determine the values of B, C, P and W. Refer to the figure below. H,0 TW F-1000 mole/ B, 8O% KOH P, S0% pure KOH filter 50% by mole KOH 10% (s0s KOH solution) 60% KOH
Chapter13: Isolation Of Eugenol From Clov
Section: Chapter Questions
Problem 9Q
Related questions
Question
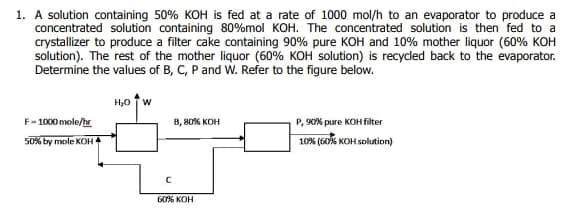
Transcribed Image Text:1. A solution containing 50% KOH is fed at a rate of 1000 mol/h to an evaporator to produce a
concentrated solution containing 80%mol KOH. The concentrated solution is then fed to a
crystallizer to produce a filter cake containing 90% pure KOH and 10% mother liquor (60% KOH
solution). The rest of the mother liquor (60% KOH solution) is recycled back to the evaporator.
Determine the values of B, C, P and W. Refer to the figure below.
H,0 TW
P, 9O% pure KOH filter
10% (s0% KOH solution)
F-100X0 mole/hr
B, 80% KOH
50% by mole KOH
60% KOH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT