4.0g of potassium hydrogen tartrate was added to 300mL distilled water. The temperature of the solution is 23.1C. The liquid was filtered and 50mL of the filtered solution was transferred to 250mL beaker, two drops of phenolpthalein was added to the 250mL beaker. The concentration of NaOH is 1.0 M that is filled in the 2mL graduate pipette, single drops of NaOH was added to the 250mL beaker until the solution turns pink and the potassium hydrogen tartrate reach the endpoint. The datd of four trials was collected. Please answer the following questions 4) calculate ksp for potassium hydrogen tartrate for each trial and average ksp for thr experiment 5) calculate the percent error in you value of ksp using reference that is found as the theoretical value. 6) why was the temperature of the saturated solution recorded?
4.0g of potassium hydrogen tartrate was added to 300mL distilled water. The temperature of the solution is 23.1C. The liquid was filtered and 50mL of the filtered solution was transferred to 250mL beaker, two drops of phenolpthalein was added to the 250mL beaker. The concentration of NaOH is 1.0 M that is filled in the 2mL graduate pipette, single drops of NaOH was added to the 250mL beaker until the solution turns pink and the potassium hydrogen tartrate reach the endpoint. The datd of four trials was collected. Please answer the following questions 4) calculate ksp for potassium hydrogen tartrate for each trial and average ksp for thr experiment 5) calculate the percent error in you value of ksp using reference that is found as the theoretical value. 6) why was the temperature of the saturated solution recorded?
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter2: Measurements And Calculations
Section: Chapter Questions
Problem 104AP: Convert 45 mi/h to m/s, showing how the units cancel appropriately.
Related questions
Question
4.0g of potassium hydrogen tartrate was added to 300mL distilled water. The temperature of the solution is 23.1C. The liquid was filtered and 50mL of the filtered solution was transferred to 250mL beaker, two drops of phenolpthalein was added to the 250mL beaker. The concentration of NaOH is 1.0 M that is filled in the 2mL graduate pipette, single drops of NaOH was added to the 250mL beaker until the solution turns pink and the potassium hydrogen tartrate reach the endpoint.
The datd of four trials was collected.
Please answer the following questions
4) calculate ksp for potassium hydrogen tartrate for each trial and average ksp for thr experiment
5) calculate the percent error in you value of ksp using reference that is found as the theoretical value.
6) why was the temperature of the saturated solution recorded?
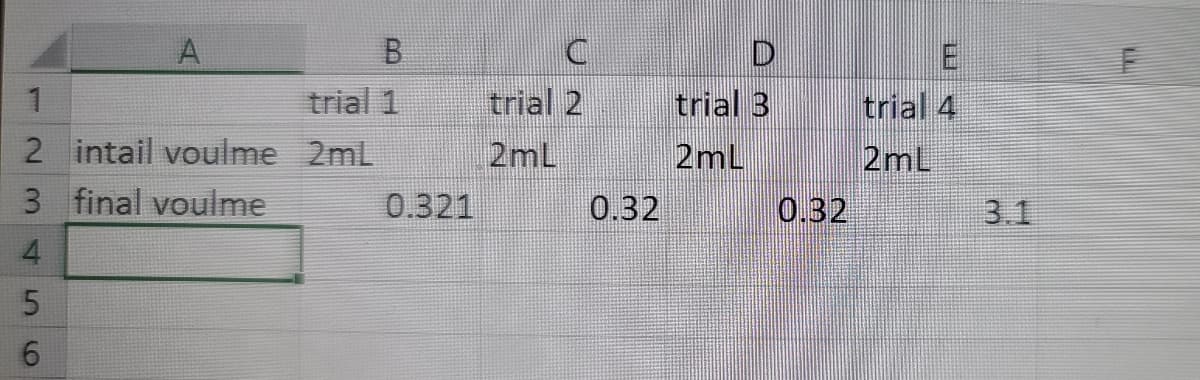
Transcribed Image Text:trial 1
trial 2
trial 3
trial 4
2mL
2mL
2 intail voulme 2mL
3 final voulme
2mL
0.321
0.32
0.32
3.1
4
B.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


