Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
ChapterL: Let's Review
SectionL.2: Making Measurements: Precision, Accuracy, Experimental Error, And Standard Deviation
Problem 1RC
Related questions
Question
Please help with chemistry scientific notation . . Solve the problem with DIMENSIONAL ANALYSIS. In proper scientific notation and using scientific notation. .
Transcribed Image Text:55
%24
31
Yanis garcia YC
Layout
References
Mailings
Review
View
Help
ernet can contain viruses. Unless you need to edit, it's safer to stay in Protected View.
Enable Editing
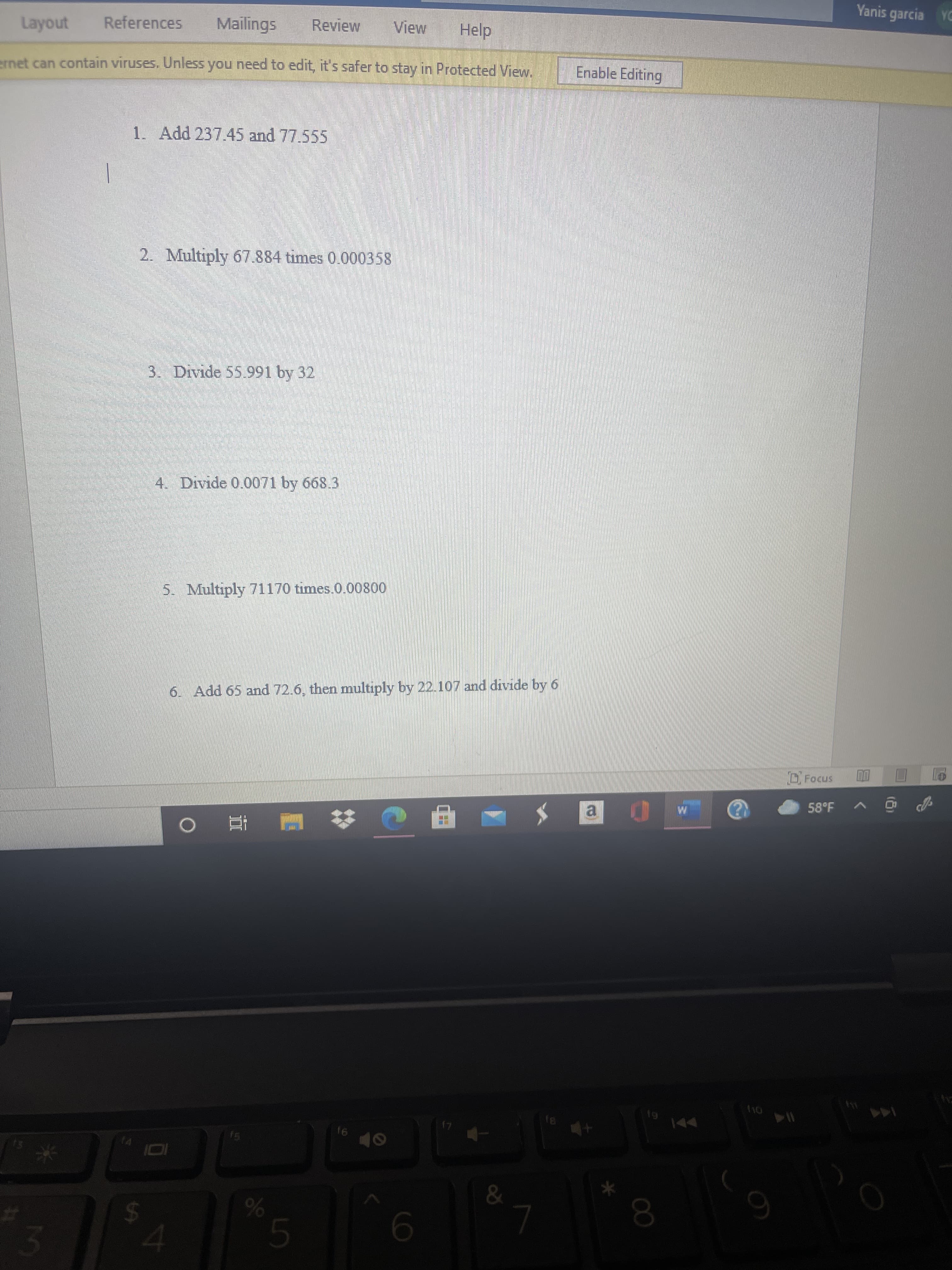
1. Add 237.45 and 77.555
2. Multiply 67.884 times 0.000358
3. Divide 55.991 by 32
4. Divide 0.0071 by 668.3
5. Multiply 71170 times.0.00800
6. Add 65 and 72.6, then multiply by 22.107 and divide by 6
O Focus
58°F
al
144
fe
114
61
91
IC
&
9-
4.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning