A certified reference material of lead in calcium carbonate is analyzed by a laboratory and found to have a lead content of 237 ng/g.237 ng/g. The reported certified value for this material is 211 ng/g.211 ng/g. What is the absolute error and the percent relative error for the method used in this analysis? absolute error: percent relative error:
A certified reference material of lead in calcium carbonate is analyzed by a laboratory and found to have a lead content of 237 ng/g.237 ng/g. The reported certified value for this material is 211 ng/g.211 ng/g. What is the absolute error and the percent relative error for the method used in this analysis? absolute error: percent relative error:
Chapter5: Errors In Chemical Analyses
Section: Chapter Questions
Problem 5.1QAP
Related questions
Question
A certified reference material of lead in calcium carbonate is analyzed by a laboratory and found to have a lead content of 237 ng/g.237 ng/g. The reported certified value for this material is 211 ng/g.211 ng/g.
What is the absolute error and the percent relative error for the method used in this analysis?
absolute error:
percent relative error:
Expert Solution
Step 1
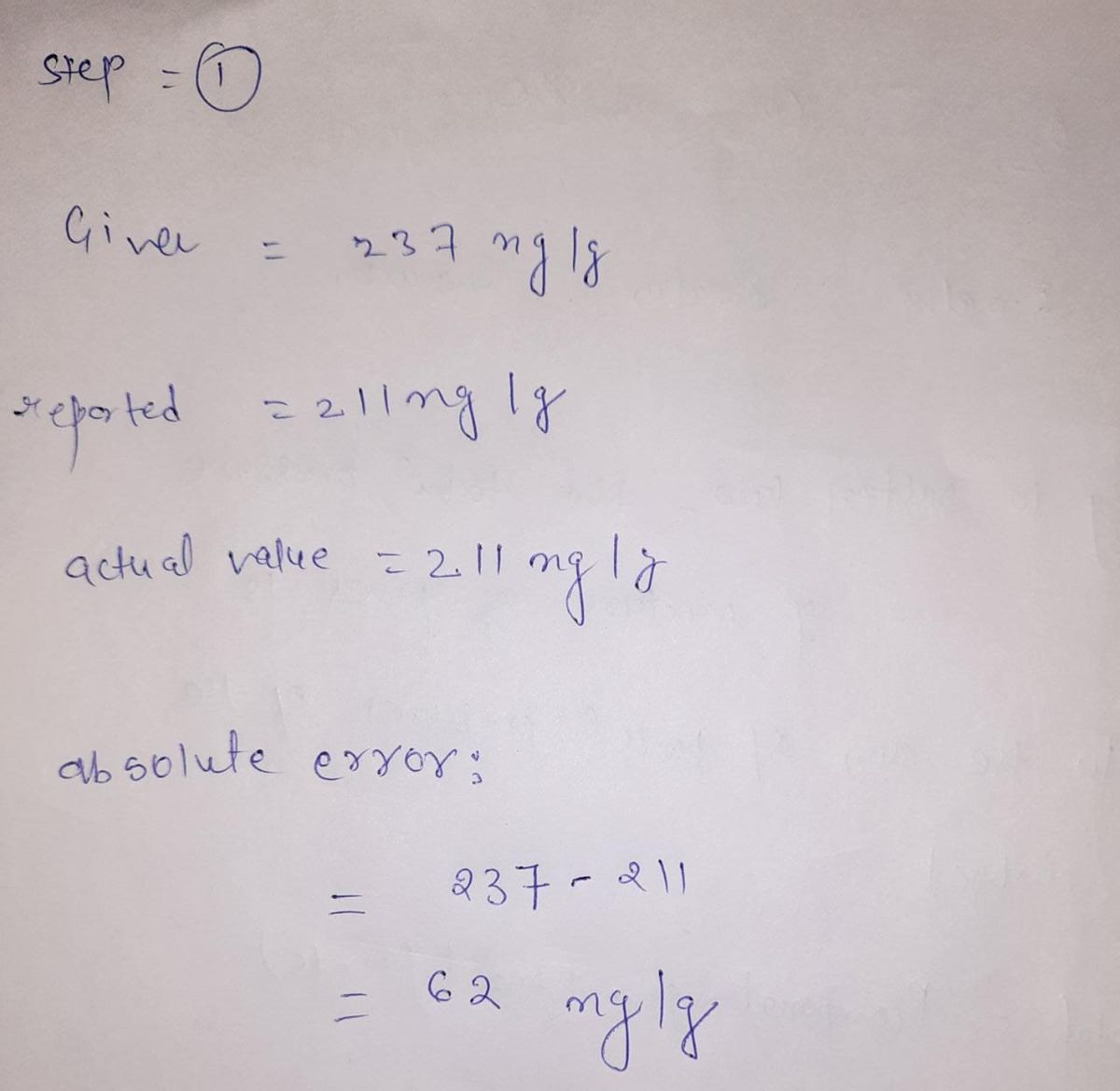
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning



Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning