silver I silver 2 silver 3 Mass of silver colored sample in grams 29659 413 349 54.155 Initial volume reading (units of mL) 20 mL 27ML Final volume reading (units of mL.) 31 mL 52mL Volume, silver material (take the difference) Density 210 2.71 2.11 Density value from Slope (with units)
silver I silver 2 silver 3 Mass of silver colored sample in grams 29659 413 349 54.155 Initial volume reading (units of mL) 20 mL 27ML Final volume reading (units of mL.) 31 mL 52mL Volume, silver material (take the difference) Density 210 2.71 2.11 Density value from Slope (with units)
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter1: Introduction To Chemistry
Section: Chapter Questions
Problem 1.10PAE: How can a liquid be distinguished from a fine powder? What type of experiment or observation might...
Related questions
Question
1
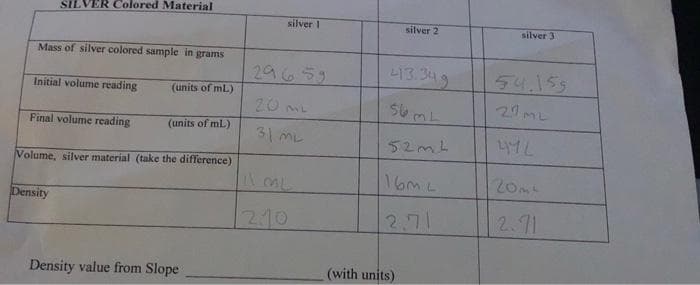
Transcribed Image Text:SILVER Colored Material
silver I
silver 2
silver 3
Mass of silver colored sample in grams
29659
L13.349
54.155
Initial volume reading
(units of mL)
20 mL
27
ML
Final volume reading
(units of mL)
31 ML
52mL
Volume, silver material (take the difference)
16ML
20mt
Density
210
2.71
2.11
Density value from Slope
(with units)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning