1. Assuming you have available both enantiomers of the amino acid valine, how would you convert the iodide A shown below to the indicated carboxylic acid? You may assume other common reagents and solvents needed are also available. enantiosel. OBn alkylation OBn H₂N CO₂H (valine)
1. Assuming you have available both enantiomers of the amino acid valine, how would you convert the iodide A shown below to the indicated carboxylic acid? You may assume other common reagents and solvents needed are also available. enantiosel. OBn alkylation OBn H₂N CO₂H (valine)
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter26: Lipids
Section26.6: Fat-soluble Vitamins
Problem BQ
Related questions
Question
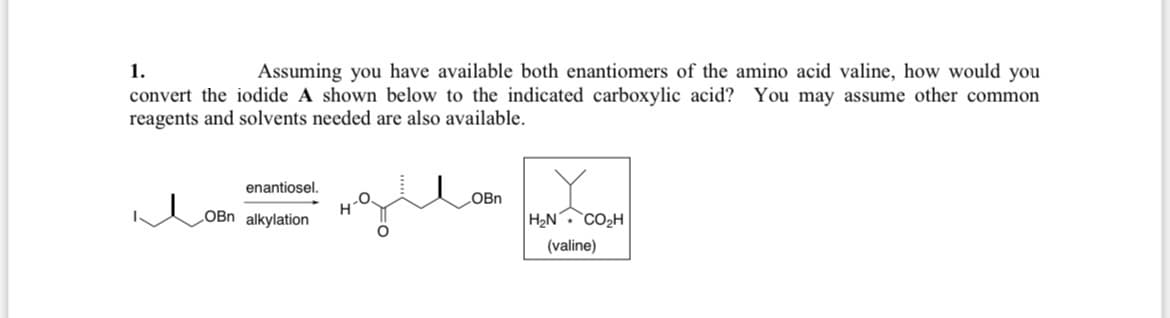
Transcribed Image Text:1.
Assuming you have available both enantiomers of the amino acid valine, how would you
convert the iodide A shown below to the indicated carboxylic acid? You may assume other common
reagents and solvents needed are also available.
enantiosel.
OBn alkylation
OBn
H₂N
CO₂H
(valine)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning



Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

