You were given an unknown mixture that contains two metal salts (A) Co(NO3)2 and (B) Cr(NO3)2 and requested to determine their concentration. The following representative information about the absorbance data were also provided. Measurements were made in a 1.0 cm quartz cells. Pure A (mol/l) 1.5 x 10-1 Pure B (mol/l) Abs @510 nm Abs @575 nm 0 0.714 0.097 0 6 x 102 0.298 0.757 Unknown conc in mixture 0.671 0.330 Unknown conc in mixture How would you go about calculating the concentration of the two salts?
You were given an unknown mixture that contains two metal salts (A) Co(NO3)2 and (B) Cr(NO3)2 and requested to determine their concentration. The following representative information about the absorbance data were also provided. Measurements were made in a 1.0 cm quartz cells. Pure A (mol/l) 1.5 x 10-1 Pure B (mol/l) Abs @510 nm Abs @575 nm 0 0.714 0.097 0 6 x 102 0.298 0.757 Unknown conc in mixture 0.671 0.330 Unknown conc in mixture How would you go about calculating the concentration of the two salts?
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter27: Gas Chromatography
Section: Chapter Questions
Problem 27.5QAP
Related questions
Question
Calculate the concentration of the two salts given their absorbances.
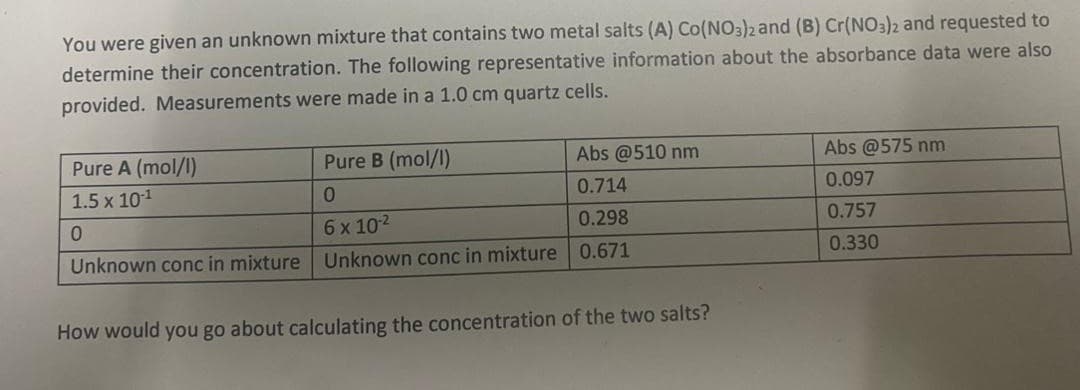
Transcribed Image Text:You were given an unknown mixture that contains two metal salts (A) Co(NO3)2 and (B) Cr(NO3)2 and requested to
determine their concentration. The following representative information about the absorbance data were also
provided. Measurements were made in a 1.0 cm quartz cells.
Pure A (mol/l)
1.5 x 10-1
Pure B (mol/l)
Abs @510 nm
Abs @575 nm
0
0.714
0.097
0
6 x 102
0.298
0.757
Unknown conc in mixture 0.671
0.330
Unknown conc in mixture
How would you go about calculating the concentration of the two salts?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 11 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

