1. Calculate the energy released by the reaction given in sample problem 12 and compare it to the energy released by the alpha decay of californium–252.
1. Calculate the energy released by the reaction given in sample problem 12 and compare it to the energy released by the alpha decay of californium–252.
ChapterU5: Fire: Energy , Thermodynamics, And Oxidation-reduction
SectionU5.116: How Absorbing: Spectroscopy
Problem 2E
Related questions
Question
1. Calculate the energy released by the reaction given in sample problem 12 and compare it to the energy released by the alpha decay of californium–252.
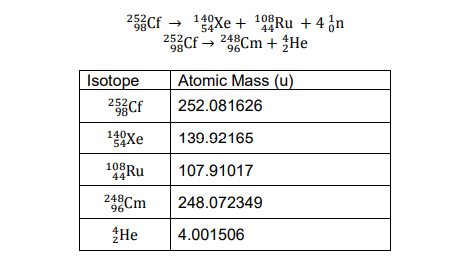
Transcribed Image Text:25Cf → 1Xe + 19Ru +4 ¿n
108Ru + 4 on
253Cf → 28Cm + He
Cm + ¿He
96
Isotope
Atomic Mass (u)
252Cf
98
252.081626
140
Xe
139.92165
108RU
44
107.91017
248Cm
96
248.072349
He
4.001506
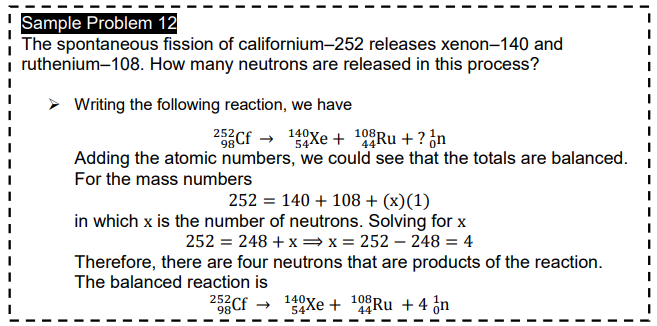
Transcribed Image Text:Sample Problem 12
The spontaneous fission of californium-252 releases xenon-140 and
i ruthenium-108. How many neutrons are released in this process?
Writing the following reaction, we have
252Cf → 140Xe + 10°Ru + ? ¿n
Adding the atomic numbers, we could see that the totals are balanced.
For the mass numbers
252 = 140 + 108 + (x)(1)
in which x is the number of neutrons. Solving for x
252 = 248 + x =x= 252 – 248 = 4
Therefore, there are four neutrons that are products of the reaction.
The balanced reaction is
252Cf
108
140y
54Xe +
Ru + 4 ¿n
98
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning



Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning