1. Calculate the percent of SiO, in the mixture by using the equation: %= part * 100 whole 2. Calculate the percent of NaCl in the in the mixture. (continued on back!) Fundamental Chemistry I 44 3. Calculate the percent recovery for the experiment.
1. Calculate the percent of SiO, in the mixture by using the equation: %= part * 100 whole 2. Calculate the percent of NaCl in the in the mixture. (continued on back!) Fundamental Chemistry I 44 3. Calculate the percent recovery for the experiment.
Chapter5: Errors In Chemical Analyses
Section: Chapter Questions
Problem 5.9QAP
Related questions
Question
Transcribed Image Text:Lab 6 Data Sheet
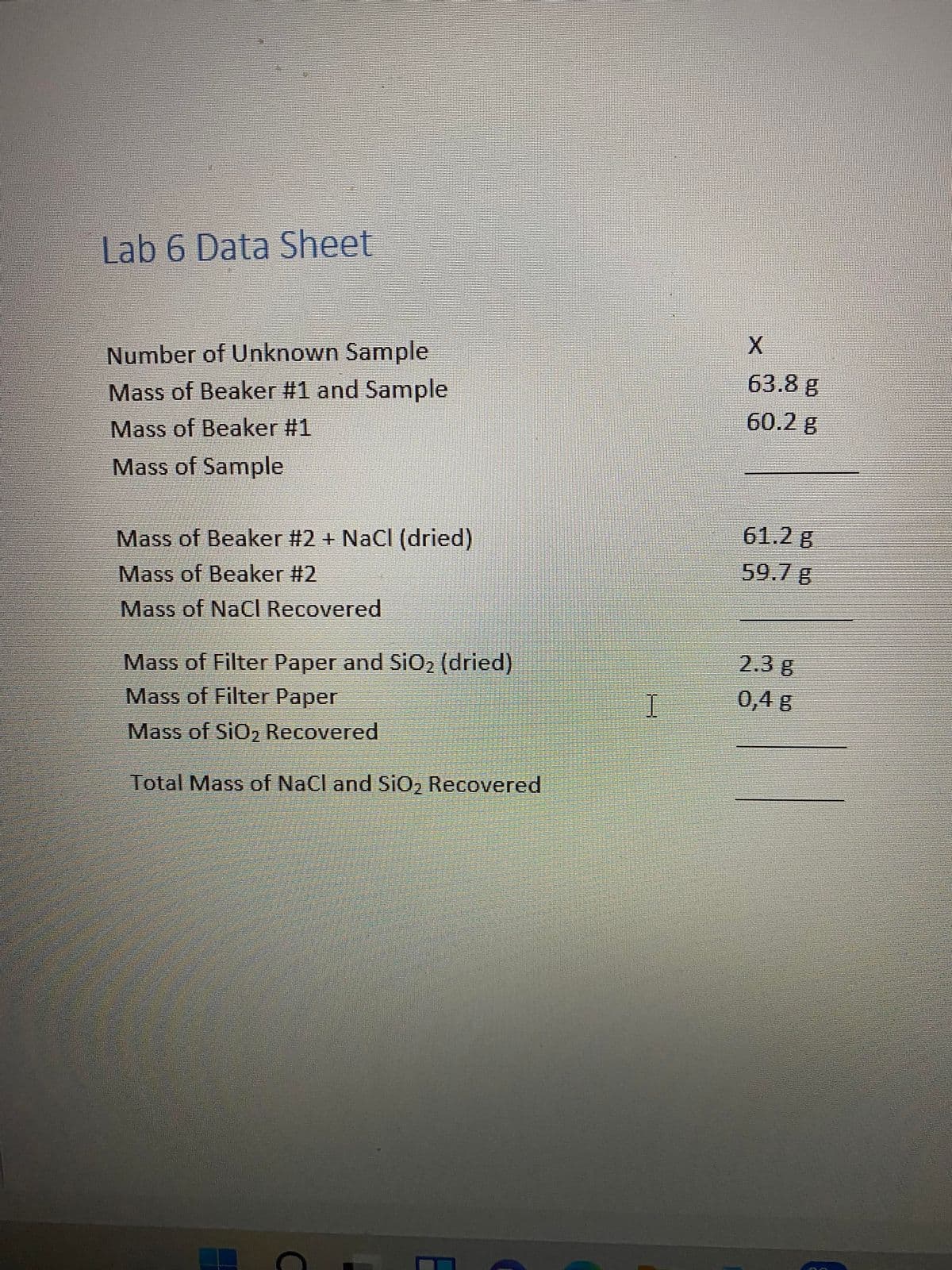
Number of Unknown Sample
Mass of Beaker #1 and Sample
63.8 g
Mass of Beaker #1
60.2 g
Mass of Sample
Mass of Beaker #2 + NaCl (dried)
61.2 g
Mass of Beaker #2
59.7 g
Mass of NaCI Recovered
Mass of Filter Paper and SiO2 (dried)
Mass of Filter Paper
2.3 g
0,4 g
Mass of SiO2 Recovered
Total Mass of NaCl and SiO2 Recovered
Transcribed Image Text:ab X,
Font
Paragraph
Styles
Calculations:
1. Calculate the percent of SiO, in the mixture by using the equation: %= part * 100
whole
2. Calculate the percent of NaCl in the in the mixture.
(continued on back!)
Fundamental Chemistry I
44
3. Calculate the percent recovery for the experiment.
4. Calculate the percent error for the separation of the mixture bv using the following equation
Text Predictions: On
* Accessibility: Investīgate
%23
99+
W
>
>
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



