1. Describe the difference between addition polymerization and conden- sation polymerization. For an example of each type of polymerization a) Give the structural formula of the starting materials b) Draw a section of the polymer chain, showing at least one repeat unit
1. Describe the difference between addition polymerization and conden- sation polymerization. For an example of each type of polymerization a) Give the structural formula of the starting materials b) Draw a section of the polymer chain, showing at least one repeat unit
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter23: Organic Polymers, Natural And Synthetic
Section: Chapter Questions
Problem 51QAP
Related questions
Question

Transcribed Image Text:1. Describe the difference between addition polymerization and conden-
sation polymerization. For an example of each type of polymerization
a) Give the structural formula of the starting materials
b) Draw a section of the polymer chain, showing at least one repeat
unit
8
2. State the product of the following chemical reactions.
a) Hydrolysis of methyl benzoate, C6H5COOCH3, with dilute sulfuric
acid.
b) Combustion of propan-1-ol.
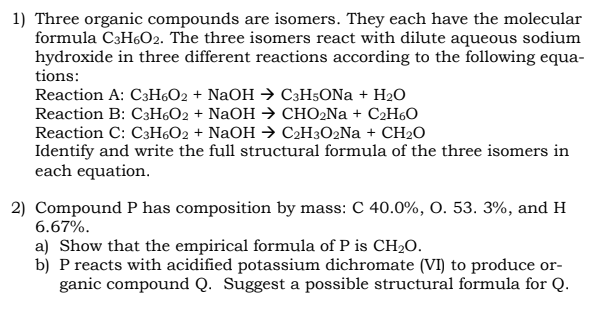
Transcribed Image Text:1) Three organic compounds are isomers. They each have the molecular
formula C3H6O2. The three isomers react with dilute aqueous sodium
hydroxide in three different reactions according to the following equa-
tions:
Reaction A: C3H6O2 + NaOH → C3H5ONa + H₂O
Reaction B: C3H6O2 + NaOH → CHO₂Na+ C₂H6O
Reaction C: C3H6O2 + NaOH → C₂H3O2Na+ CH₂O
Identify and write the full structural formula of the three isomers in
each equation.
2) Compound P has composition by mass: C 40.0%, O. 53. 3%, and H
6.67%.
a) Show that the empirical formula of P is CH₂O.
b) P reacts with acidified potassium dichromate (VI) to produce or-
ganic compound Q. Suggest a possible structural formula for Q.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning