1. Determine the amount of energy required to melt 15.0 grams of ice. 2a. If a 2000g block of metal LOST 3120 J of heat energy as it cooled from 212 degrees C to 200 degrees C, what is the specific heat of the metal 2b. What is the element ?
1. Determine the amount of energy required to melt 15.0 grams of ice. 2a. If a 2000g block of metal LOST 3120 J of heat energy as it cooled from 212 degrees C to 200 degrees C, what is the specific heat of the metal 2b. What is the element ?
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter10: Energy
Section: Chapter Questions
Problem 38QAP: In the “Chemistry in Focus” segment Firewalking: Magic or Science?, it is claimed that one reason...
Related questions
Question
100%
1. Determine the amount of energy required to melt 15.0 grams of ice.
2a. If a 2000g block of metal LOST 3120 J of heat energy as it cooled from 212 degrees C to 200 degrees C, what is the specific heat of the metal
2b. What is the element ?
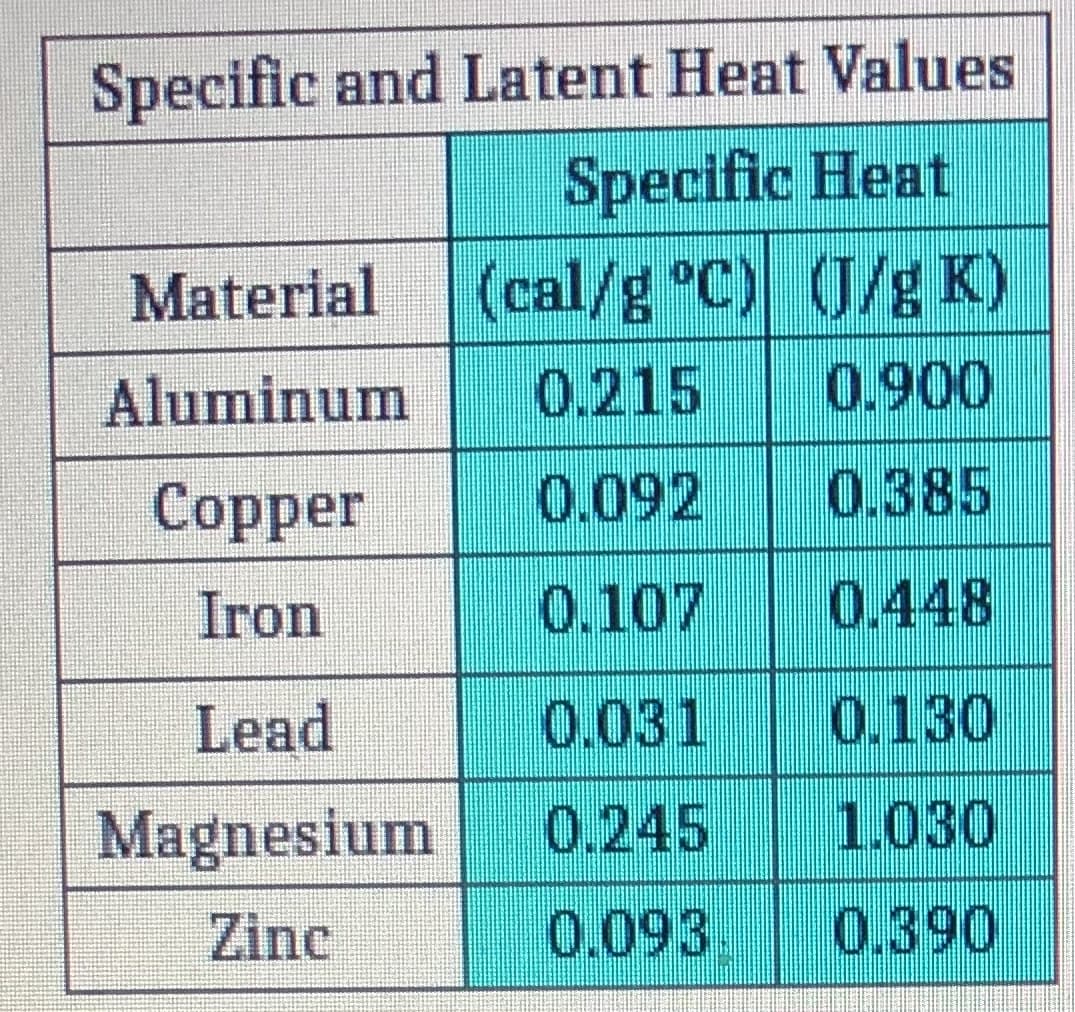
Transcribed Image Text:Specific and Latent Heat Values
Specific Heat
(cal/g °C) (J/g K)
0.215
Material
Aluminum
0.900
Copper
0.092
0.385
Iron
0.107
0.448
Lead
0.031
0.130
Magnesium
0.245
1.030
Zinc
0.093.
0.390
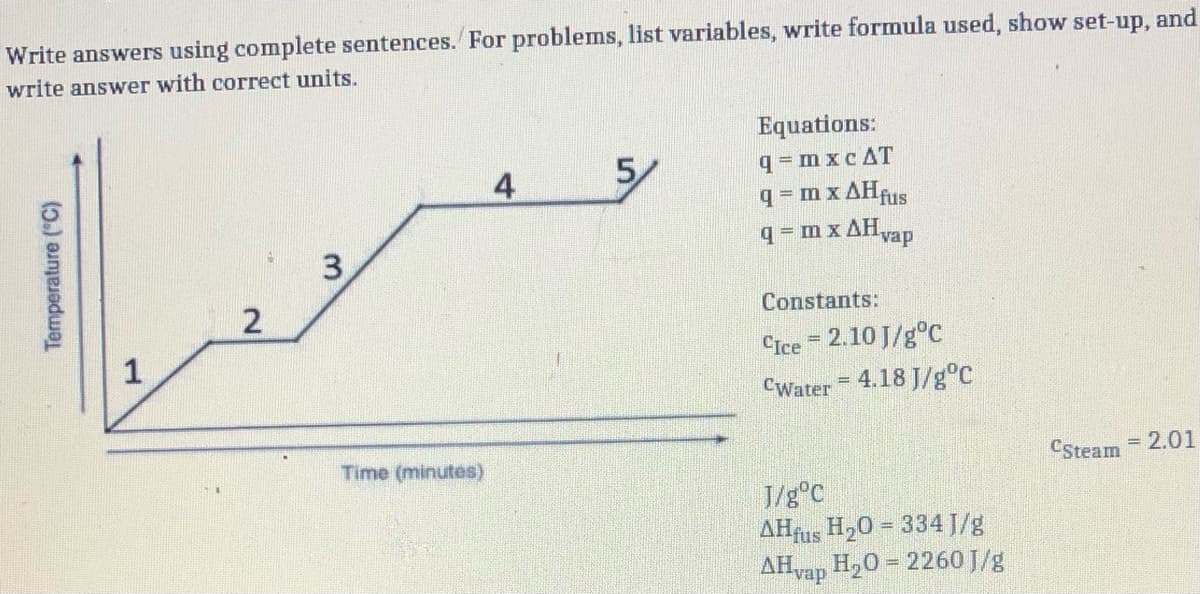
Transcribed Image Text:Write answers using complete sentences. For problems, list variables, write formula used, show set-up, and
write answer with correct units.
Equations:
q= m xc AT
q m x AHAIS
4
5/
q = m x AHvap
Constants:
1
CIce = 2.10 J/g°C
%3D
CWater = 4.18 J/g°C
Time (minutes)
CSteam
= 2.01
J/g°C
AHfus H20 = 334 J/g
AHvap H20 = 2260J/g
Temperature ("C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT