1. Direction: Complete the table by providing the missing information. Atomic Mass Elements Number number Number Number Number (Z) (A) of p of n of e K 19 39 19 20 19 Mg 12 24 12 12 12 15 31 15 16 15 Si 14 28 14 14 14 Ar 18 40 18 22 18 2. Balance the Nuclear Equation. ' 13AI +"2 He 27 1 on + 30 15P 44 20Ca +4H 1421Sc + 1 on 253 99ES +“2 He 258 101MD 239 4Pu +42He 247 e6Cm
1. Direction: Complete the table by providing the missing information. Atomic Mass Elements Number number Number Number Number (Z) (A) of p of n of e K 19 39 19 20 19 Mg 12 24 12 12 12 15 31 15 16 15 Si 14 28 14 14 14 Ar 18 40 18 22 18 2. Balance the Nuclear Equation. ' 13AI +"2 He 27 1 on + 30 15P 44 20Ca +4H 1421Sc + 1 on 253 99ES +“2 He 258 101MD 239 4Pu +42He 247 e6Cm
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter20: Nuclear Chemistry
Section: Chapter Questions
Problem 20.62QP
Related questions
Question
Hello! Can someone check if my following answers (in orange text) are correct? Thank you!
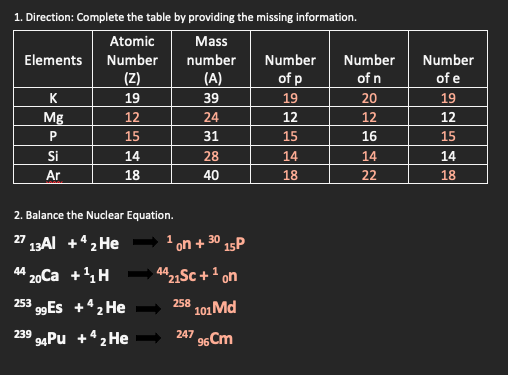
Transcribed Image Text:1. Direction: Complete the table by providing the missing information.
Atomic
Mass
Elements
Number
number
Number
Number
Number
(Z)
(A)
of p
of n
of e
K
19
39
19
20
19
Mg
12
24
12
12
12
15
31
15
16
15
Si
14
28
14
14
14
Ar
18
40
18
22
18
2. Balance the Nuclear Equation.
' 13AI +*2 He
27
1 on + 30 15P
44 20Ca +4H
1421Sc + 1 on
253 99ES +“2 He
258 101 Md
239 4Pu +42He
247 e6Cm
Expert Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
