1. Calculate the energy released by the reaction given in sample problem 12 and compare it to the energy released by the alpha decay of californium-252. 252 Cf → 120Xe + *44! 98 54Xe + 19Ru +4 in 108 25Cf → 29Cm + He 248Cm + He Isotope Atomic Mass (u) 252Cf 98 252.081626 140y 54Xe 139.92165 108 44RU 107.91017 248 960 248.072349 He 4.001506
1. Calculate the energy released by the reaction given in sample problem 12 and compare it to the energy released by the alpha decay of californium-252. 252 Cf → 120Xe + *44! 98 54Xe + 19Ru +4 in 108 25Cf → 29Cm + He 248Cm + He Isotope Atomic Mass (u) 252Cf 98 252.081626 140y 54Xe 139.92165 108 44RU 107.91017 248 960 248.072349 He 4.001506
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter25: Nuclear Chemistry
Section: Chapter Questions
Problem 68IL
Related questions
Question
Please answer no. 1 and 2 in the attached image.
Note: For No. 1, Sample problem 12 is also attached in the second image.
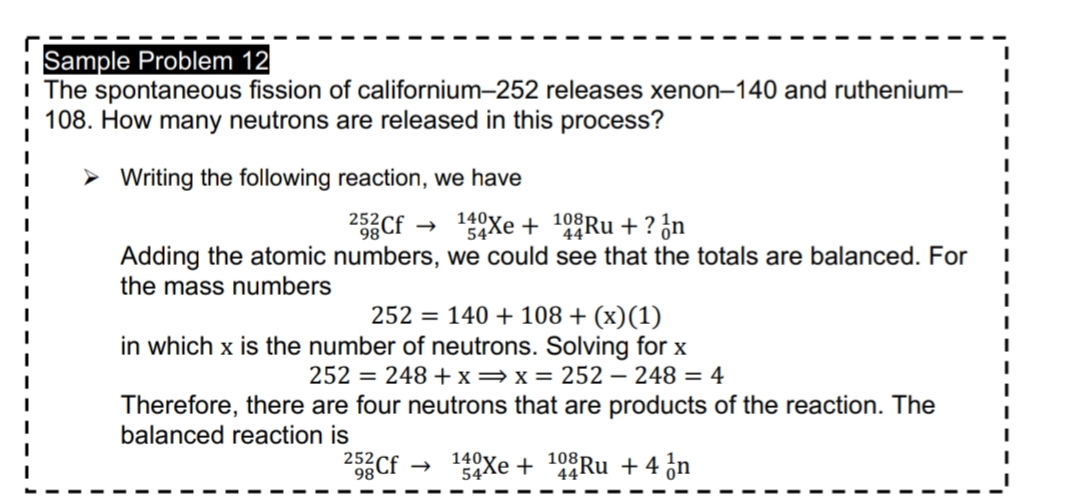
Transcribed Image Text:Sample Problem 12
I The spontaneous fission of californium-252 releases xenon–140 and ruthenium-
108. How many neutrons are released in this process?
> Writing the following reaction, we have
252
2Cf
140Xe + 10°Ru + ? ¿n
544
Adding the atomic numbers, we could see that the totals are balanced. For
the mass numbers
252 = 140 + 108 + (x)(1)
in which x is the number of neutrons. Solving for x
252 = 248 +x=x= 252 – 248 = 4
Therefore, there are four neutrons that are products of the reaction. The
balanced reaction is
252,
98
140y
54Xe + 44RU +4 ¿n
108
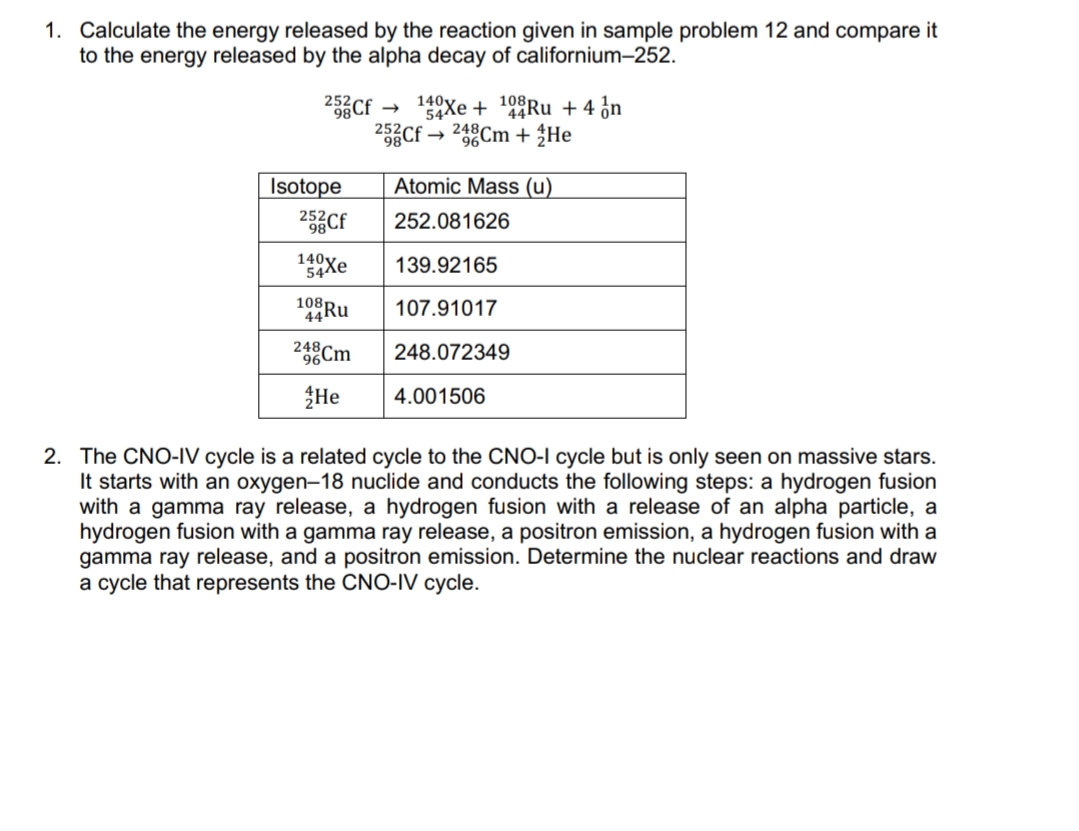
Transcribed Image Text:1. Calculate the energy released by the reaction given in sample problem 12 and compare it
to the energy released by the alpha decay of californium-252.
2Cf
140y.
252
54Xe + 9Ru +4 ¿n
25Cf → 248Cm + ŽHe
Isotope
Atomic Mass (u)
25Cf
252.081626
140Xe
139.92165
108
107.91017
24Cm
248.072349
He
4.001506
2. The CNO-IV cycle is a related cycle to the CNO-I cycle but is only seen on massive stars.
It starts with an oxygen-18 nuclide and conducts the following steps: a hydrogen fusion
with a gamma ray release, a hydrogen fusion with a release of an alpha particle, a
hydrogen fusion with a gamma ray release, a positron emission, a hydrogen fusion with a
gamma ray release, and a positron emission. Determine the nuclear reactions and draw
a cycle that represents the CNO-IV cycle.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning