1. Explanation of properties of
2. Differences of Intermolecular forces (London Dispersion Forces, dipole-dipole Forces, Ion-Dipole Forces, and Hydrogen Bond)
3. In medical industry, Medical devices use adhesives as one example of application of Intermolecular Forces of Attraction. Adhesives are used extensively in th medical world, from simple plasters to advanced medical applications. Adhesives are integral to the production of paper tissues and nappies, allow tablets to be protected from the effects of moisture and allow wounds to be dressed. Trans dermal patches, such as nicotine patch, permit a controlled delivery of nicotine into the bloodstream to help smokers quit. What are other line of work that applies the Intermolecular forces of Attraction. At least one example and its use in the certain field of work.
4. Prediction of the type of Intermolecular forces that will be formed with the same kind of (a-c) and different kinds of molecules (d-e) (ATTACHED IMAGE)
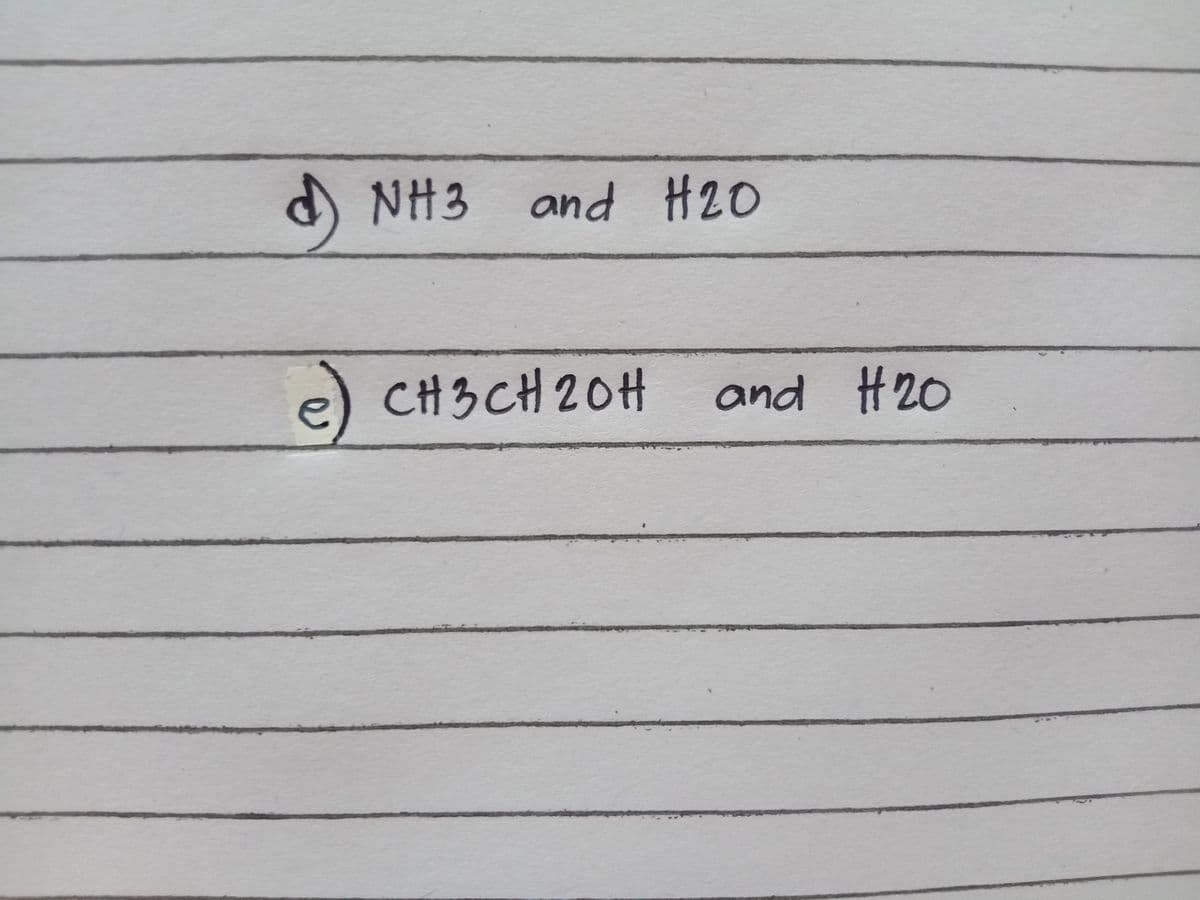
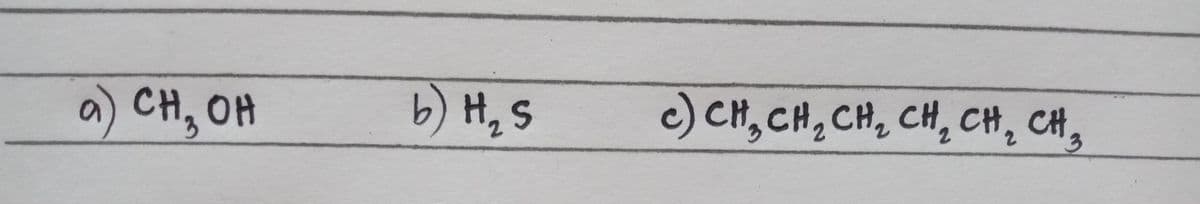
Step by step
Solved in 4 steps with 1 images









