100. Draw ionic Lewis structures for KF and CaC. Use the in- formation and the method in the previous problem to pre- dict which of these two ionic solids has the higher melting point.
100. Draw ionic Lewis structures for KF and CaC. Use the in- formation and the method in the previous problem to pre- dict which of these two ionic solids has the higher melting point.
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter4: Molecular Structure And Orbitals
Section: Chapter Questions
Problem 9Q: Which of the following statements is/are true? Correct the false statements. a. The molecules SeS3....
Related questions
Question
#100
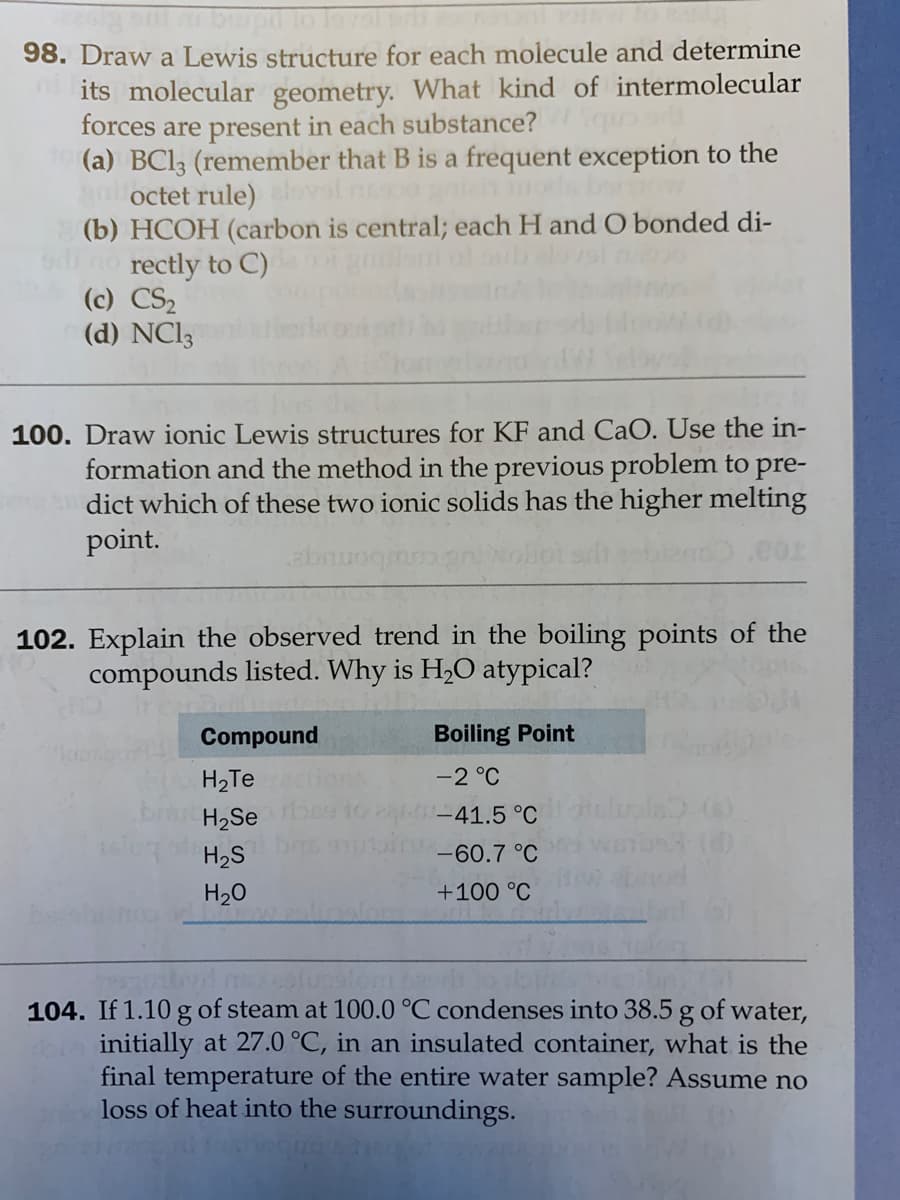
Transcribed Image Text:98. Draw a Lewis structure for each molecule and determine
its molecular geometry. What kind of intermolecular
forces are present in each substance?
(a) BC13 (remember that B is a frequent exception to the
gaoctet rule) ovol n
(b) HCOH (carbon is central; each H and O bonded di-
no rectly to C)
(c) CS2
(d) NCI3
100. Draw ionic Lewis structures for KF and CaO. Use the in-
formation and the method in the previous problem to pre-
dict which of these two ionic solids has the higher melting
point.
abn
102. Explain the observed trend in the boiling points of the
compounds listed. Why is H,O atypical?
Compound
Boiling Point
H2Te
-2 °C
H2Se
-41.5 °C
elupla.(s)
H2S
-60.7 °C
H20
+100 °C
104. If 1.10 g of steam at 100.0 °C condenses into 38.5 g of water,
initially at 27.0 °C, in an insulated container, what is the
final temperature of the entire water sample? Assume no
loss of heat into the surroundings.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning