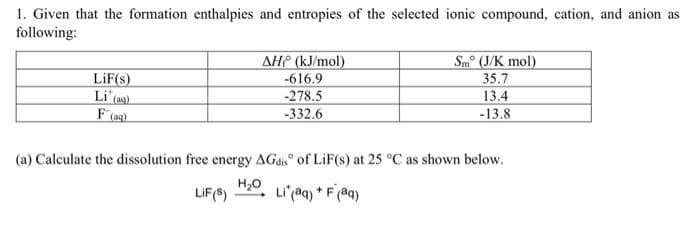
1. Given that the formation enthalpies and entropies of the selected ionic compound, cation, and anion as following: AHP (kJ/mol) LiF(s) Li (ag) Sm° (J/K mol) 35.7 13.4 -13.8 -616.9 -278.5 -332.6 (a) Calculate the dissolution free energy AGdis of LiF(s) at 25 °C as shown below. H20 LIF(8) Li (aq) * F (@q)
1. Given that the formation enthalpies and entropies of the selected ionic compound, cation, and anion as following: AHP (kJ/mol) LiF(s) Li (ag) Sm° (J/K mol) 35.7 13.4 -13.8 -616.9 -278.5 -332.6 (a) Calculate the dissolution free energy AGdis of LiF(s) at 25 °C as shown below. H20 LIF(8) Li (aq) * F (@q)
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter66: The Synthesis Of Natural Products: The Sex Attractant Of The Cockroach And Camphor
Section: Chapter Questions
Problem 3Q
Related questions
Question
Solve it asap
Transcribed Image Text:1. Given that the formation enthalpies and entropies of the selected ionic compound, cation, and anion as
following:
AHP (kJ/mol)
-616.9
-278.5
-332.6
Sm° (J/K mol)
35.7
13.4
-13.8
LiF(s)
Li (ag)
(a) Calculate the dissolution free energy AGds of LiF(s) at 25 °C as shown below.
H20
LIF(8)
Li'(aq) * F(aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning