1) Determine the solubility product constant (Ksp) of borax, a slightly soluble sodium salt, in water at two different temperatures and evaluate the enthalpy, entropy and Gibbs free energy change for dissolving borax in water. Materials and reagents: Borax (Na2B4O5(OH)4· 10H2O) Na2CO3 0.1 M HCl solution Bromocresol green indicator Bromothymol blue indicator 500 ml Erlenmeyer flasks (10 per student) Beakers (2 per student) Magnetic stirrer and bar (1 per student) Distilled water Ice-water bath Thermometer (1 per student) 50 ml burette (1 per student) Procedure: Preparation of the Borax-Borate Equilibrium Mixtures: These solutions will be prepared beforehand. Take about 60 mL of each solution. Make sure each student performs at least one titration. To two separate 500mL Erlenmeyer flasks containing magnetic stirring bars add about 20g of borax and 400mL of distilled water. Designate one flask as the room temperature system and one as the ice-water system. Stir the room temperature mixture gently on a magnetic stirrer (with no heating) for at least ten minutes. Shut off the stirrer, place a thermometer in the flask and leave it undisturbed to allow the excess undissolved borax to settle to the bottom. The solution portion should become clear after a few minutes. Place the second Erlenmeyer flask in an ice-water bath (be sure to use enough ice) on a magnetic stirrer and stir the mixture with no heating for at least 20 minutes. Shut off the stirrer, place a thermometer in the flask and allow the undissolved borax to settle to the bottom. This mixture temperature should be close to 5ºC and the solution portion should become clear. Keep the flask undisturbed in the ice bath until aliquots are removed for titration. Standardization of 0.1 M HCl Titrating Solution: While the borax mixtures are stirring, standardize by titration using already prepared 0.1 M HCl. Use three 5-mL portions to rinse a clean 50 mL burette with the HCl solution and then fill the burette with the HCl solution. Accurately weigh three approximately 0.15 g portions of Na2CO3 to 0.01 g into separate clean but not necessarily dry Erlenmeyer flasks. To each flask add 3 drops of bromocresol green indicator and 50mL of distilled water. The solution will turn blue. Titrate each portion of sodium carbonate to the yellow-green endpoint. Do more than three titrations if your instructor says your results are not consistent. Use the titration data to calculate the actual concentration of your HCl solution according to the following equation: Ksp of Borax at Room Temperature: Record the temperature of the room temperature borax mixture. Without disturbing the solid at the bottom, carefully decant about 60 mL of the borate solution into a clean and dry beaker and pipet three 10.00 mL aliquots into three separate clean but not necessarily dry Erlenmeyer flasks. Add 20 mL of distilled water and 3 drops of bromothymol blue indicator to each flask. The solutions should turn blue. Titrate each sample with your standardized HCl solution until the solution changes from blue to yellow-green. Use your best three titrations to evaluate the Ksp of borax at room temperature. Ksp of Borax at Ice Temperature: Read the temperature of the iced borax mixture and carefully decant about 60 mL of the solution into a clean and dry beaker. Pipet three 10.00 mL aliquots into three separate Erlenmeyer flasks. To each flask, add 20 mL of distilled water and 3 drops of bromothymol blue indicator. Titrate each sample with HCl until the color changes to yellow-green. Use your best three titrations to evaluate the Ksp of borax at ice temperature.
1)
Determine the solubility product constant (Ksp) of borax, a slightly soluble sodium salt, in water at two different temperatures and evaluate the enthalpy, entropy and Gibbs free energy change for dissolving borax in water.
Materials and reagents:
Borax (Na2B4O5(OH)4· 10H2O)
Na2CO3
0.1 M HCl solution
Bromocresol green indicator
Bromothymol blue indicator
500 ml Erlenmeyer flasks (10 per student)
Beakers (2 per student)
Magnetic stirrer and bar (1 per student)
Distilled water
Ice-water bath
Thermometer (1 per student)
50 ml burette (1 per student)
Procedure:
Preparation of the Borax-Borate Equilibrium Mixtures:
These solutions will be prepared beforehand.
Take about 60 mL of each solution. Make sure each student performs at least one titration.
- To two separate 500mL Erlenmeyer flasks containing magnetic stirring bars add about 20g of borax and 400mL of distilled water. Designate one flask as the room temperature system and one as the ice-water system.
- Stir the room temperature mixture gently on a magnetic stirrer (with no heating) for at least ten minutes. Shut off the stirrer, place a thermometer in the flask and leave it undisturbed to allow the excess undissolved borax to settle to the bottom.
- The solution portion should become clear after a few minutes. Place the second Erlenmeyer flask in an ice-water bath (be sure to use enough ice) on a magnetic stirrer and stir the mixture with no heating for at least 20 minutes.
- Shut off the stirrer, place a thermometer in the flask and allow the undissolved borax to settle to the bottom. This mixture temperature should be close to 5ºC and the solution portion should become clear. Keep the flask undisturbed in the ice bath until aliquots are removed for titration.
Standardization of 0.1 M HCl Titrating Solution:
- While the borax mixtures are stirring, standardize by titration using already prepared 0.1 M HCl. Use three 5-mL portions to rinse a clean 50 mL burette with the HCl solution and then fill the burette with the HCl solution.
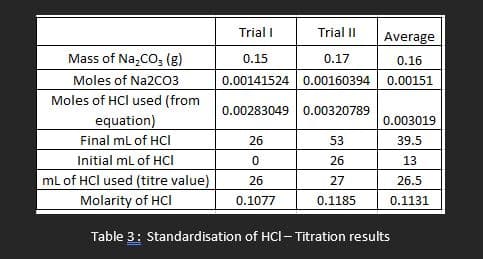
- Accurately weigh three approximately 0.15 g portions of Na2CO3 to 0.01 g into separate clean but not necessarily dry Erlenmeyer flasks. To each flask add 3 drops of bromocresol green indicator and 50mL of distilled water. The solution will turn blue. Titrate each portion of sodium carbonate to the yellow-green endpoint.
- Do more than three titrations if your instructor says your results are not consistent. Use the titration data to calculate the actual concentration of your HCl solution according to the following equation:
Ksp of Borax at Room Temperature:
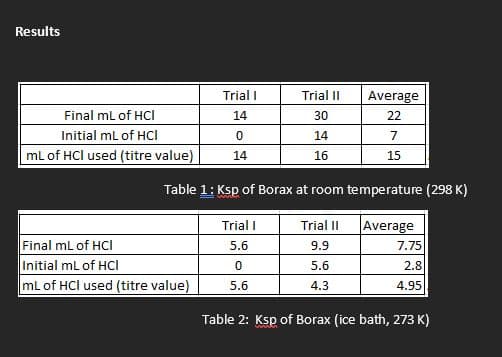
- Record the temperature of the room temperature borax mixture. Without disturbing the solid at the bottom, carefully decant about 60 mL of the borate solution into a clean and dry beaker and pipet three 10.00 mL aliquots into three separate clean but not necessarily dry Erlenmeyer flasks.
- Add 20 mL of distilled water and 3 drops of bromothymol blue indicator to each flask. The solutions should turn blue.
- Titrate each sample with your standardized HCl solution until the solution changes from blue to yellow-green. Use your best three titrations to evaluate the Ksp of borax at room temperature.
Ksp of Borax at Ice Temperature: Read the temperature of the iced borax mixture and carefully decant about 60 mL of the solution into a clean and dry beaker. Pipet three 10.00 mL aliquots into three separate Erlenmeyer flasks.
- To each flask, add 20 mL of distilled water and 3 drops of bromothymol blue indicator.
- Titrate each sample with HCl until the color changes to yellow-green.
- Use your best three titrations to evaluate the Ksp of borax at ice temperature.
Step by step
Solved in 10 steps with 31 images






