1. Given the following cell diagram at 298K: Ag) | AgBr) | NaBr(ag, 0.050M) 1) NaCl(aq, 0.125M) |Cl2(g, 0.88bar) Pt(s) (a) Write the net chemical equation for the reaction. (b) Determine AGº, ASº, AH°, and E° for this electrochemical reaction. (c) Calculate the cell potential (E) under the cell conditions. 2. Consider redox reaction 2Cu+ (aq) → Cu(s) + Cu²+( * (aq) operating under non-standard-state conditions. Suppose that the cell's reaction is multiplied by 2. What effect does this have on each of the following quantities in the Nernst equation: Ecell RT = E° cell In Q? nF a. E cell b. Edell c. 2 d. n
1. Given the following cell diagram at 298K: Ag) | AgBr) | NaBr(ag, 0.050M) 1) NaCl(aq, 0.125M) |Cl2(g, 0.88bar) Pt(s) (a) Write the net chemical equation for the reaction. (b) Determine AGº, ASº, AH°, and E° for this electrochemical reaction. (c) Calculate the cell potential (E) under the cell conditions. 2. Consider redox reaction 2Cu+ (aq) → Cu(s) + Cu²+( * (aq) operating under non-standard-state conditions. Suppose that the cell's reaction is multiplied by 2. What effect does this have on each of the following quantities in the Nernst equation: Ecell RT = E° cell In Q? nF a. E cell b. Edell c. 2 d. n
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter32: Voltaic Cell Measurements
Section: Chapter Questions
Problem 2ASA
Related questions
Question
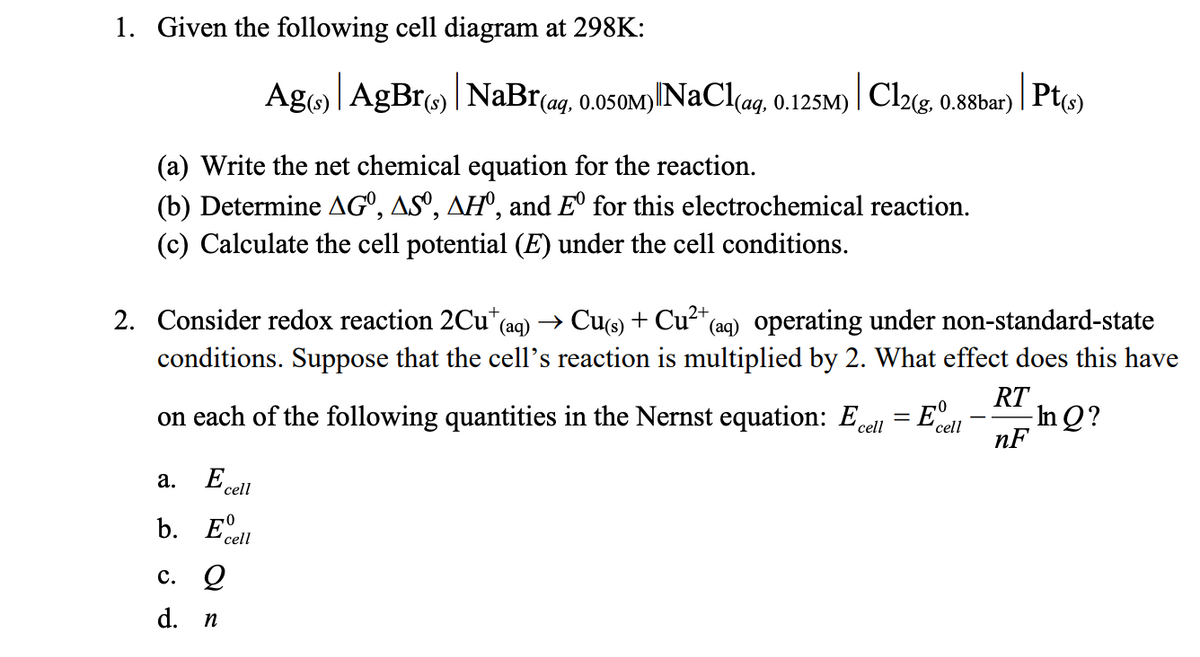
Transcribed Image Text:1. Given the following cell diagram at 298K:
Ag) | AgBr) | NaBr(ag, 0.050M)
1) NaCl(aq, 0.125M)
|Cl2(g,
0.88bar)
Pt(s)
(a) Write the net chemical equation for the reaction.
(b) Determine AGº, ASº, AH°, and E° for this electrochemical reaction.
(c) Calculate the cell potential (E) under the cell conditions.
2. Consider redox reaction 2Cu+ (aq) → Cu(s) + Cu²+(
* (aq) operating under non-standard-state
conditions. Suppose that the cell's reaction is multiplied by 2. What effect does this have
on each of the following quantities in the Nernst equation: Ecell
RT
=
E°
cell
In Q?
nF
a.
E cell
b. Edell
c. 2
d. n
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,
