1. Here we have two molecules. First one is the salicylic acid and the second one is the acetyl salicylic acid. You can see the pKa values under the molecules. According to the pKa values Salicylic acid is more acidic. Explain why. COOH COOH COCH 3 Salicylic acid Acetyl Salicylic acid pKa: 2.97 pКa: 3.50
1. Here we have two molecules. First one is the salicylic acid and the second one is the acetyl salicylic acid. You can see the pKa values under the molecules. According to the pKa values Salicylic acid is more acidic. Explain why. COOH COOH COCH 3 Salicylic acid Acetyl Salicylic acid pKa: 2.97 pКa: 3.50
Chapter20: Carboxylic Acids And Nitriles
Section20.3: Biological Acids And The Henderson–hasselbalch Equation
Problem 5P: Calculate the percentages of dissociated and undissociated forms present in the following solutions:...
Related questions
Question
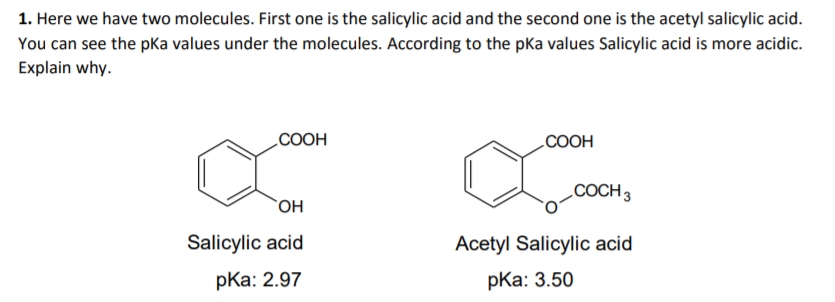
Transcribed Image Text:1. Here we have two molecules. First one is the salicylic acid and the second one is the acetyl salicylic acid.
You can see the pKa values under the molecules. According to the pka values Salicylic acid is more acidic.
Explain why.
COOH
COOH
COCH 3
OH
Salicylic acid
Acetyl Salicylic acid
pKa: 2.97
pKa: 3.50
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning



Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning