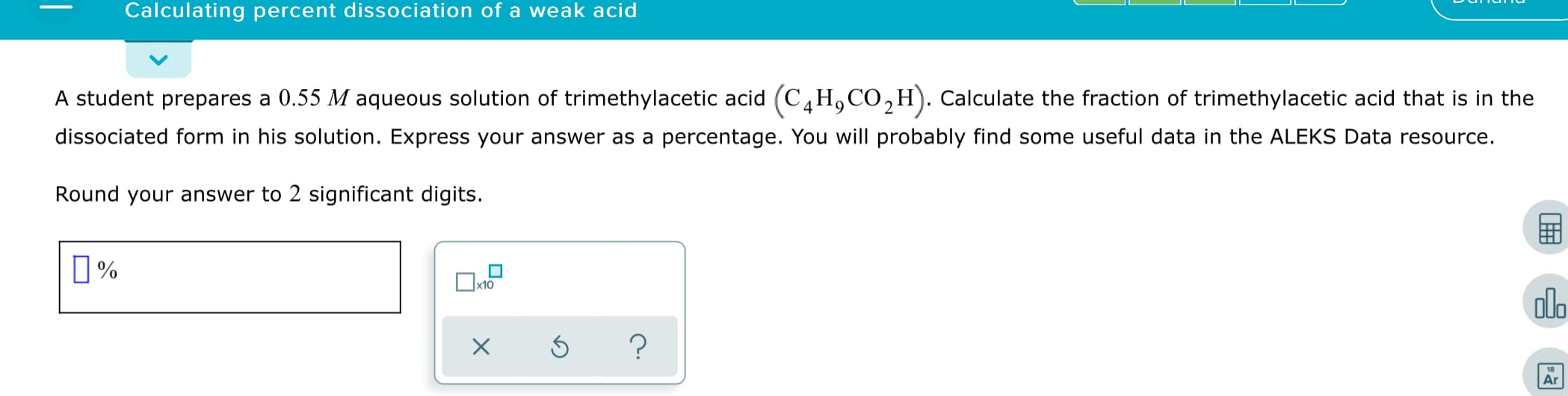
Calculating percent dissociation of a weak acid A student prepares a 0.55 M aqueous solution of trimethylacetic acid (C,H,CO,H). Calculate the fraction of trimethylacetic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. x10 olo Ar [-] pK, of weak acids at 25°C Name Formula pKą1 pKą2 pКаз acetic acid CH3CO2H 4.756 benzoic acid CH5CO2H 4.204 butanoic acid Сзн,со2н 4.83 4-chlorobutanoic acid C3H,CICO,H 4.52 crotonic acid C3H5CO2H 4.69 охalic acid H2C2O4 1.25 3.81 phosphoric acid НзРО4 2.16 7.21 12.32 propionic acid C2H5CO2H 4.87 sulfuric acid H2SO4 strong acid 1.99 trimethylacetic acid C4H9CO2H 5.03
Calculating percent dissociation of a weak acid A student prepares a 0.55 M aqueous solution of trimethylacetic acid (C,H,CO,H). Calculate the fraction of trimethylacetic acid that is in the dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource. Round your answer to 2 significant digits. x10 olo Ar [-] pK, of weak acids at 25°C Name Formula pKą1 pKą2 pКаз acetic acid CH3CO2H 4.756 benzoic acid CH5CO2H 4.204 butanoic acid Сзн,со2н 4.83 4-chlorobutanoic acid C3H,CICO,H 4.52 crotonic acid C3H5CO2H 4.69 охalic acid H2C2O4 1.25 3.81 phosphoric acid НзРО4 2.16 7.21 12.32 propionic acid C2H5CO2H 4.87 sulfuric acid H2SO4 strong acid 1.99 trimethylacetic acid C4H9CO2H 5.03
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%
Transcribed Image Text:Calculating percent dissociation of a weak acid
A student prepares a 0.55 M aqueous solution of trimethylacetic acid (C,H,CO,H). Calculate the fraction of trimethylacetic acid that is in the
dissociated form in his solution. Express your answer as a percentage. You will probably find some useful data in the ALEKS Data resource.
Round your answer to 2 significant digits.
x10
olo
Ar
![[-] pK, of weak acids at 25°C
Name
Formula
pKą1
pKą2
pКаз
acetic acid
CH3CO2H
4.756
benzoic acid
CH5CO2H
4.204
butanoic acid
Сзн,со2н
4.83
4-chlorobutanoic acid
C3H,CICO,H
4.52
crotonic acid
C3H5CO2H
4.69
охalic acid
H2C2O4
1.25
3.81
phosphoric acid
НзРО4
2.16
7.21
12.32
propionic acid
C2H5CO2H
4.87
sulfuric acid
H2SO4
strong acid
1.99
trimethylacetic acid
C4H9CO2H
5.03](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Feb286130-1ee5-4c42-a58a-074772cd1e39%2F1a405141-fcdc-41d2-8ca0-fc8e6e93d16d%2F0ffa45w.jpeg&w=3840&q=75)
Transcribed Image Text:[-] pK, of weak acids at 25°C
Name
Formula
pKą1
pKą2
pКаз
acetic acid
CH3CO2H
4.756
benzoic acid
CH5CO2H
4.204
butanoic acid
Сзн,со2н
4.83
4-chlorobutanoic acid
C3H,CICO,H
4.52
crotonic acid
C3H5CO2H
4.69
охalic acid
H2C2O4
1.25
3.81
phosphoric acid
НзРО4
2.16
7.21
12.32
propionic acid
C2H5CO2H
4.87
sulfuric acid
H2SO4
strong acid
1.99
trimethylacetic acid
C4H9CO2H
5.03
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY