1. If a sample contains 100 g of a radioactive isotope, how much will be left after 3 half lives? 2. If a sample contains 50g of a radioactive isotope, how much will be left after 2 half lives? 3. If a sample contains 20g of an isotope that has a half life of 1000 years. How much will be left after 2000years?
1. If a sample contains 100 g of a radioactive isotope, how much will be left after 3 half lives? 2. If a sample contains 50g of a radioactive isotope, how much will be left after 2 half lives? 3. If a sample contains 20g of an isotope that has a half life of 1000 years. How much will be left after 2000years?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter18: Nuclear Reactions
Section: Chapter Questions
Problem 80QAP: Carbon-14 (C-14) with a half-life of 5730 years decays to nitrogen-14 (N-14). A sample of carbon...
Related questions
Question
need help
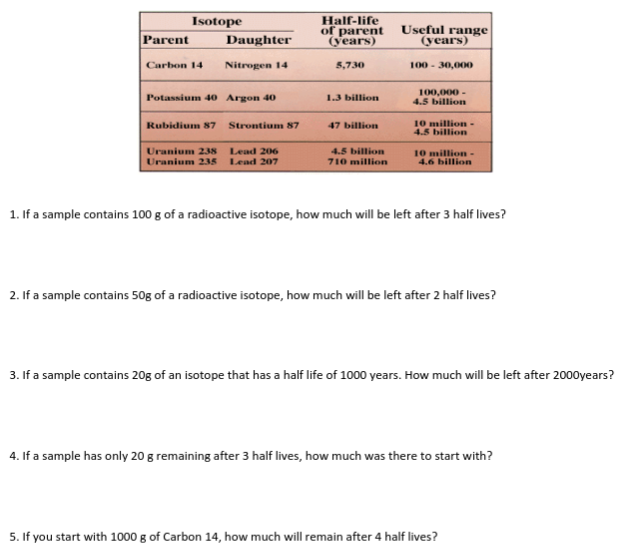
Transcribed Image Text:Half-life
of parent
(years)
Isotope
Parent
Daughter
Useful range
(уears)
Nitrogen 14
5,730
100 - 30,000
Carbon 14
100,000 -
4.5 billion
Potassium 40 Argon 40
1.3 billion
10 million -
4.5 billion
Rubidium 87 Strontium 87
47 billion
Uranium 238 Lead 206
Uranium 235
4.5 billion
710 million
10 million-
4.6 billion
Lead 207
1. If a sample contains 100 g of a radioactive isotope, how much will be left after 3 half lives?
2. If a sample contains 50g of a radioactive isotope, how much will be left after 2 half lives?
3. If a sample contains 20g of an isotope that has a half life of 1000 years. How much will be left after 2000years?
4. If a sample has only 20 g remaining after 3 half lives, how much was there to start with?
5. If you start with 1000 g of Carbon 14, how much will remain after 4 half lives?

Transcribed Image Text:5. If you start with 1000 g of Carbon 14, how much will remain after 4 half lives?
6. Assume you start with 10,000 Carbon 14 atoms. How many will remain after:
2 half lives?
1 half life?
3 half lives?.
7. What is the half life of Potassium 40?,
8. If a fossil originally contained 100,000 Potassium 40 atoms and it now contains only 12,500, how old is the
fossil?
Use the following diagram to answer # 9-11:
O minutes
10 minutes
20 minutes
30 minutes
9. What is the half life of the element in the picture?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning