1. If the reaction below is initially at equilibrium, and then each of the following changes are made, predict which direction the reaction rate will be fastest until equilibrium is once again established: forward, reverse, or no change. H2(g) + Cl2(g) 2 HCl(g) a. the concentration of H₂ is increased forward b. the concentration of HCl is decreased forward c. the concentration of Cl₂ is decreased reverse
1. If the reaction below is initially at equilibrium, and then each of the following changes are made, predict which direction the reaction rate will be fastest until equilibrium is once again established: forward, reverse, or no change. H2(g) + Cl2(g) 2 HCl(g) a. the concentration of H₂ is increased forward b. the concentration of HCl is decreased forward c. the concentration of Cl₂ is decreased reverse
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter16: Acids And Bases
Section: Chapter Questions
Problem 65AP: . The concepts of acid-base equilibria were developed in this chapter for aqueous solutions (in...
Related questions
Question
100%
Can you help me to explain step by step with number 1 part A, number 1 part B, and number 1 part C? Can you explain it to me?
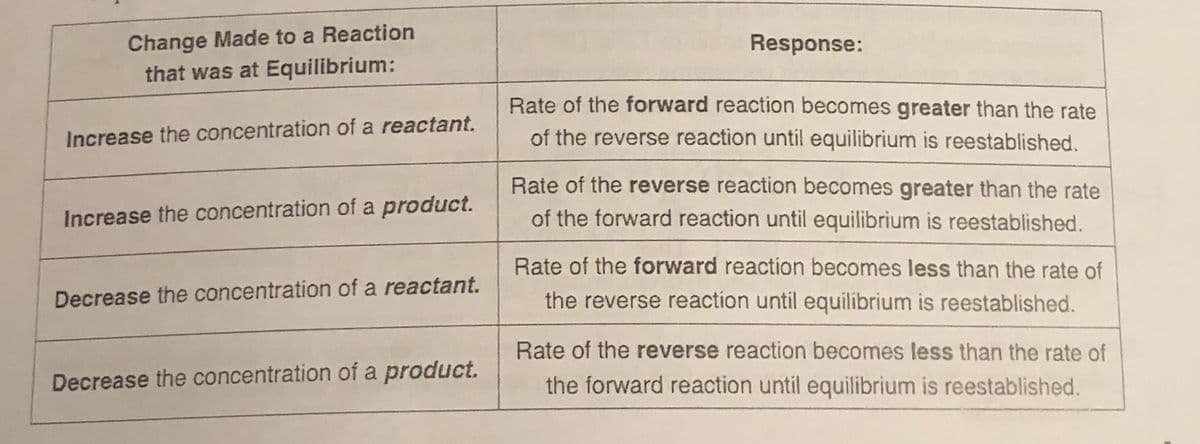
Transcribed Image Text:Change Made to a Reaction
that was at Equilibrium:
Increase the concentration of a reactant.
Increase the concentration of a product.
Decrease the concentration of a reactant.
Decrease the concentration of a product.
Response:
Rate of the forward reaction becomes greater than the rate
of the reverse reaction until equilibrium is reestablished.
Rate of the reverse reaction becomes greater than the rate
of the forward reaction until equilibrium is reestablished.
Rate of the forward reaction becomes less than the rate of
the reverse reaction until equilibrium is reestablished.
Rate of the reverse reaction becomes less than the rate of
the forward reaction until equilibrium is reestablished.
![● Definition of equilibrium
• Keq: law of mass action
●
Le Chatelier's Principle
Topics:
●
●
●
Properties of acids & bases
Brønstead-Lowry Acids & Bases definition
Kw & calculating [H3O+], [OH-], pH
1. If the reaction below is initially at equilibrium, and then each of the following changes are made, predict
which direction the reaction rate will be fastest until equilibrium is once again established: forward,
reverse, or no change.
H2(g) + Cl2(g) 2 HCl(g)
a. the concentration of H₂ is increased forward
b. the concentration of HCl is decreased forward
c. the concentration of Cl₂ is decreased versc
2. If the reaction below is initially at equilibrium, and then more NH4* is added, predict which direction the
reaction rate will be fastest until equilibrium is once again established: forward, reverse, or no
change. Forward
If more Hu is added
If more wHyt addel, forwa rol NH4* (aq) + H₂O (1) NH3 (aq) +
Hut reactant
reaction fast until equilibrium
reestablished
-1
[30+ (aq)
3. Define (a) reversible reaction and (b) equilibrium.
A) Reversible reaction is
produce original reactant,
one where the products of the reaction can themselves react to
B.) Equilibrium: Amount of reactant reach a balance istay there,
4. For the reaction below: reverse reaction take place exactly same rate in both direction
Reaction takes place in both direction but over all effect forward's
2 NO2 (g) + O2 (g) 2 NO3 (g)
a. Write the law of mass action (the equilibrium expression for Keq)
Keq = [03]
кед
[c]c[d] Products
Aja (B]b reactants
[0₂] [NO₂]²
b. If gas concentrations are as follows, 2.10 M NO2, 1.75 M O2, and 1.00 M NO3, calculate Keq
1.00m]
0.130x102
(1.00m)
0.1295
Keq=
(2.10m) ³x (675m) 0.130m²
(4.41m x1.75m) 0.130
c. Based on the Keq value that you calculated in part b, are the reactants or products predominant
(predominant means that there is a greater amount present)?
Keq is a less than 1, then equilibrium will shift to
reverse direction.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fb60ce3d5-7de7-48ff-b6dc-19fe3a1e14b8%2F3d5992a6-3532-4487-8f36-ddd74dff7ef7%2Fcppcd8m_processed.jpeg&w=3840&q=75)
Transcribed Image Text:● Definition of equilibrium
• Keq: law of mass action
●
Le Chatelier's Principle
Topics:
●
●
●
Properties of acids & bases
Brønstead-Lowry Acids & Bases definition
Kw & calculating [H3O+], [OH-], pH
1. If the reaction below is initially at equilibrium, and then each of the following changes are made, predict
which direction the reaction rate will be fastest until equilibrium is once again established: forward,
reverse, or no change.
H2(g) + Cl2(g) 2 HCl(g)
a. the concentration of H₂ is increased forward
b. the concentration of HCl is decreased forward
c. the concentration of Cl₂ is decreased versc
2. If the reaction below is initially at equilibrium, and then more NH4* is added, predict which direction the
reaction rate will be fastest until equilibrium is once again established: forward, reverse, or no
change. Forward
If more Hu is added
If more wHyt addel, forwa rol NH4* (aq) + H₂O (1) NH3 (aq) +
Hut reactant
reaction fast until equilibrium
reestablished
-1
[30+ (aq)
3. Define (a) reversible reaction and (b) equilibrium.
A) Reversible reaction is
produce original reactant,
one where the products of the reaction can themselves react to
B.) Equilibrium: Amount of reactant reach a balance istay there,
4. For the reaction below: reverse reaction take place exactly same rate in both direction
Reaction takes place in both direction but over all effect forward's
2 NO2 (g) + O2 (g) 2 NO3 (g)
a. Write the law of mass action (the equilibrium expression for Keq)
Keq = [03]
кед
[c]c[d] Products
Aja (B]b reactants
[0₂] [NO₂]²
b. If gas concentrations are as follows, 2.10 M NO2, 1.75 M O2, and 1.00 M NO3, calculate Keq
1.00m]
0.130x102
(1.00m)
0.1295
Keq=
(2.10m) ³x (675m) 0.130m²
(4.41m x1.75m) 0.130
c. Based on the Keq value that you calculated in part b, are the reactants or products predominant
(predominant means that there is a greater amount present)?
Keq is a less than 1, then equilibrium will shift to
reverse direction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
