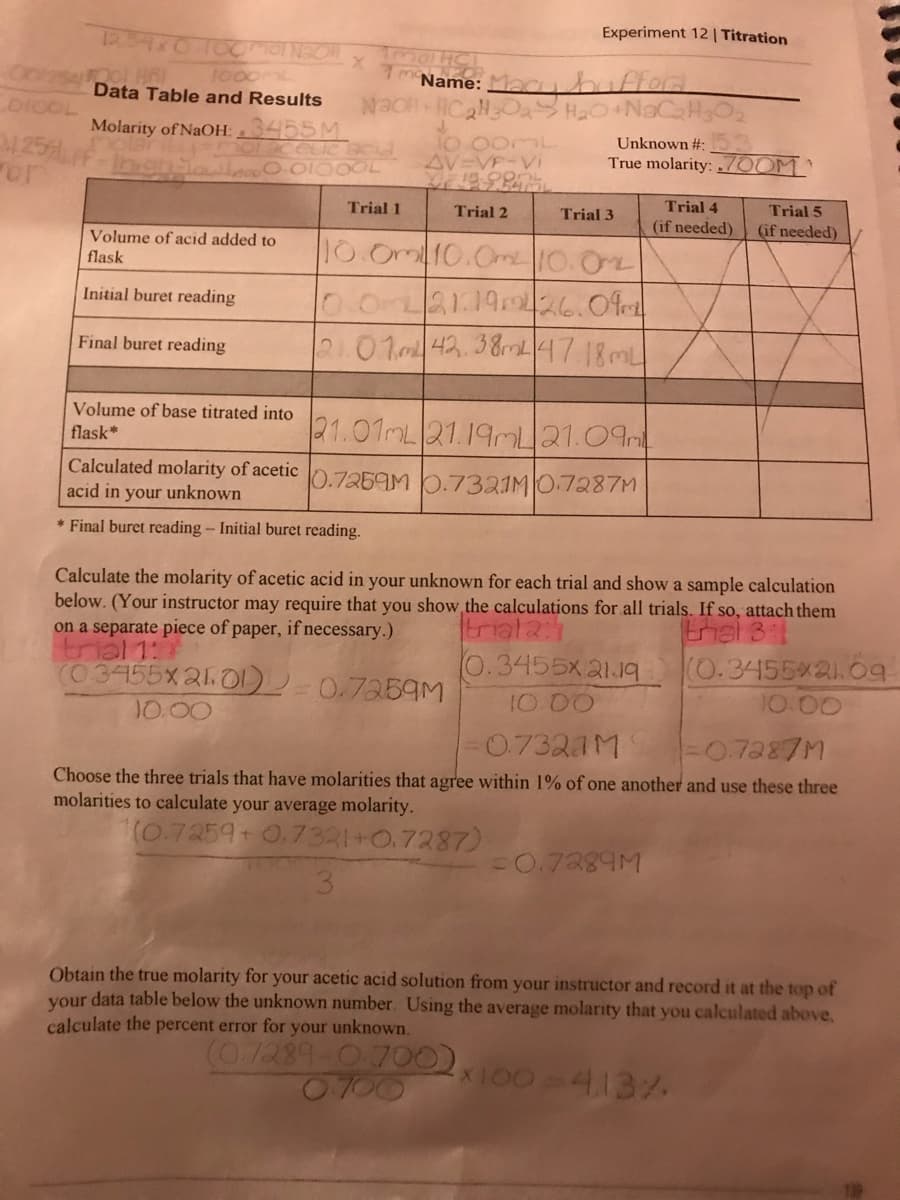
1. If you get to the end point of your titration and wait a few hours, you'll find that the color has disappeared. What has happened? Write the chemical equations for the reactions involved in this delayed color change. (Hint: See the note at the end of Step 12 in the Procedure Section of this experiment.) 2. Why do we titrate into a flask and not a beaker? 3. What effect would each of the following errors have on the determined concentration of th unknown acid? Would the calculated result be erroneously high, erroneously low, or unaffected a You forgot to condition the buret and it contained some deionized water before you added the NaOH. Circle one: too low / unaffected / too high b. The Erlenmeyer flask was wet with deionized water before you added the acid to it. Circle one: too low / unaffected / too high C. You forgot to condition the pipet before using it to transfer the acid to the flask. Circle one: too low / unaffected / too high / depends d. The final buret reading was mistakenly recorded too high (e.g., recorded as 35.00 m instead of the actual 30.00 mL). Circle one: too low / unaffected/too high Based on the true molarity of your acetic acid unknown, and assuming that its density solution is 1.01 g/mL, calculate the mass percent of acetic acid in your unknown.
Ionic Equilibrium
Chemical equilibrium and ionic equilibrium are two major concepts in chemistry. Ionic equilibrium deals with the equilibrium involved in an ionization process while chemical equilibrium deals with the equilibrium during a chemical change. Ionic equilibrium is established between the ions and unionized species in a system. Understanding the concept of ionic equilibrium is very important to answer the questions related to certain chemical reactions in chemistry.
Arrhenius Acid
Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions. Keeping it similar to the general acid properties, Arrhenius acid also neutralizes bases and turns litmus paper into red.
Bronsted Lowry Base In Inorganic Chemistry
Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with.

Step by step
Solved in 5 steps






