1. If you get to the end point of your titration and wait a few hours, you'll find that the color has disappeared. What has happened? Write the chemical equations for the reactions involved in this delayed color change. (Hint: See the note at the end of Step 12 in the Procedure Section of this experiment.)
1. If you get to the end point of your titration and wait a few hours, you'll find that the color has disappeared. What has happened? Write the chemical equations for the reactions involved in this delayed color change. (Hint: See the note at the end of Step 12 in the Procedure Section of this experiment.)
Chapter31: Introduction To Analytical Separations
Section: Chapter Questions
Problem 31.17QAP
Related questions
Question
Help please

Transcribed Image Text:3.
de33520 m Experiment 12 | Titration
Post-Lab Questions
1. If you get to the end point of your titration and wait a few hours, you'll find that the color has
disappeared. What has happened? Write the chemical equations for the reactions involved in
this delayed color change. (Hint: See the note at the end of Step 12 in the Procedure Section of
this experiment.)
2. Why do we titrate into a flask and not a beaker?
Name:
a
What effect would each of the following errors have on the determined concentration of the
unknown acid? Would the calculated result be erroneously high, erroneously low, or unaffected?
C.
You forgot to condition the buret and it contained some deionized water before you
added the NaOH. Circle one: too low / unaffected / too high
b. The Erlenmeyer flask was wet with deionized water before you added the acid to it.
Circle one: too low / unaffected / too high
You forgot to condition the pipet before using it to transfer the acid to the flask.
Circle one: too low / unaffected / too high / depends
d. The final buret reading was mistakenly recorded too high (e.g., recorded as 35.00 ml
instead of the actual 30.00 mL). Circle one: too low / unaffected / too high
4. Based on the true molarity of your acetic acid unknown, and assuming that its density of the
solution is 1.01 g/mL, calculate the mass percent of acetic acid in your unknown.
Transcribed Image Text:0.00/254
0.0100L
12.54x0 10omanson
TMOTHCL
OOL HAI
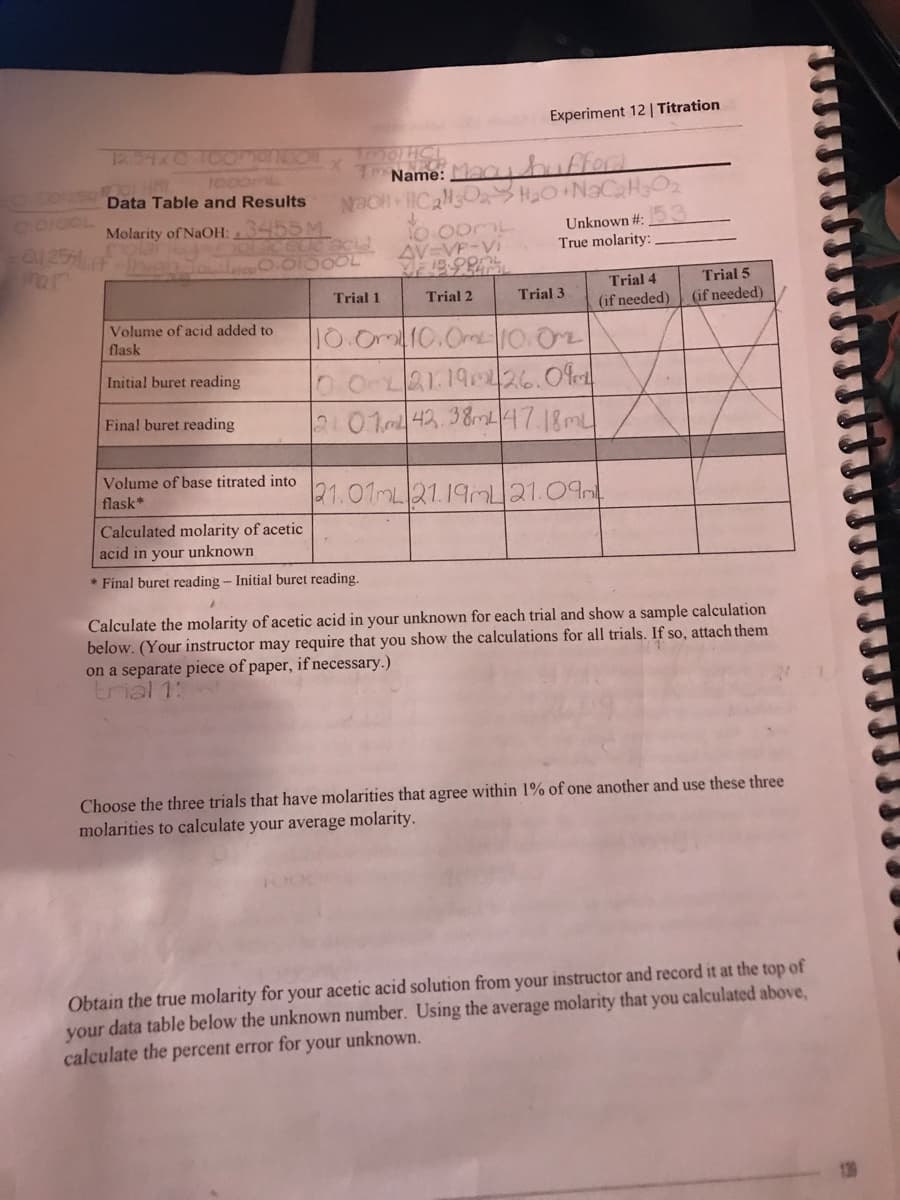
7 Name: Macy hufford
Data Table and Results Nao C₂30₂ H₂O+NaC₂H₂O₂
Molarity of NaOH:.3455 M
0.25% F
Par
olaccuc acid
0.01000L
Volume of acid added to
flask
Initial buret reading
Final buret reading
Volume of base titrated into
flask*
Trial 1
Experiment 12 | Titration
10.00ML
AV=VP-Vi
Trial 2
10.0m 10.0m 10.0
Calculated molarity of acetic
acid in your unknown
*Final buret reading - Initial buret reading.
Unknown #:
True molarity:
Trial 3
0.01 21:19426.09
21.07m 42.38m4 47.18ml
21.01mL 21.19mL 21.09
Trial 4
(if needed)
Trial 5
(if needed)
Calculate the molarity of acetic acid in your unknown for each trial and show a sample calculation
below. (Your instructor may require that you show the calculations for all trials. If so, attach them
on a separate piece of paper, if necessary.)
trial 1:
Choose the three trials that have molarities that agree within 1% of one another and use these three
molarities to calculate your average molarity.
Obtain the true molarity for your acetic acid solution from your instructor and record it at the top of
your data table below the unknown number. Using the average molarity that you calculated above,
calculate the percent error for your unknown.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

