1. Lead (II) nitrate in acidic solution reacts with zinc metal to form solid lead and zinc nitrate. Balance the redox reaction between Pb(NO3)2 and Zn occurring under acidic conditions. 2. Selenium metal reacts with Chromium(III) hydroxide in basic conditions to produce chromium metal and Selenium trioxide (overall negative 2 charge when aqueous). Balance the redox reaction between Se and Cr(OH)3 occurring in basic conditions. 3. Suppose I want to want to build a voltaic cell that has a cell potential of 3.75 V what two metal/ion combinations would I use? Give the chemical reaction for this redox process. a. What half reaction would occur at the cathode and what half reaction would occur at the anode? 4. What is the difference between a voltaic cell and an electrolytic cell? 5. How do you know if a reaction is a redox reaction? 6. Is the decomposition of hydrogen peroxide from lab 9 (2H2Ozaq) → 2H2O) + O2(8) a redox reaction? Why or why not?
1. Lead (II) nitrate in acidic solution reacts with zinc metal to form solid lead and zinc nitrate. Balance the redox reaction between Pb(NO3)2 and Zn occurring under acidic conditions. 2. Selenium metal reacts with Chromium(III) hydroxide in basic conditions to produce chromium metal and Selenium trioxide (overall negative 2 charge when aqueous). Balance the redox reaction between Se and Cr(OH)3 occurring in basic conditions. 3. Suppose I want to want to build a voltaic cell that has a cell potential of 3.75 V what two metal/ion combinations would I use? Give the chemical reaction for this redox process. a. What half reaction would occur at the cathode and what half reaction would occur at the anode? 4. What is the difference between a voltaic cell and an electrolytic cell? 5. How do you know if a reaction is a redox reaction? 6. Is the decomposition of hydrogen peroxide from lab 9 (2H2Ozaq) → 2H2O) + O2(8) a redox reaction? Why or why not?
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter19: Principles Of Chemical Reactivity: Electron Transfer Reactions
Section: Chapter Questions
Problem 100GQ: Copper(I) ion disproportionates to copper metal and copper(ll) ion. (See Study Question 99.) 2...
Related questions
Question
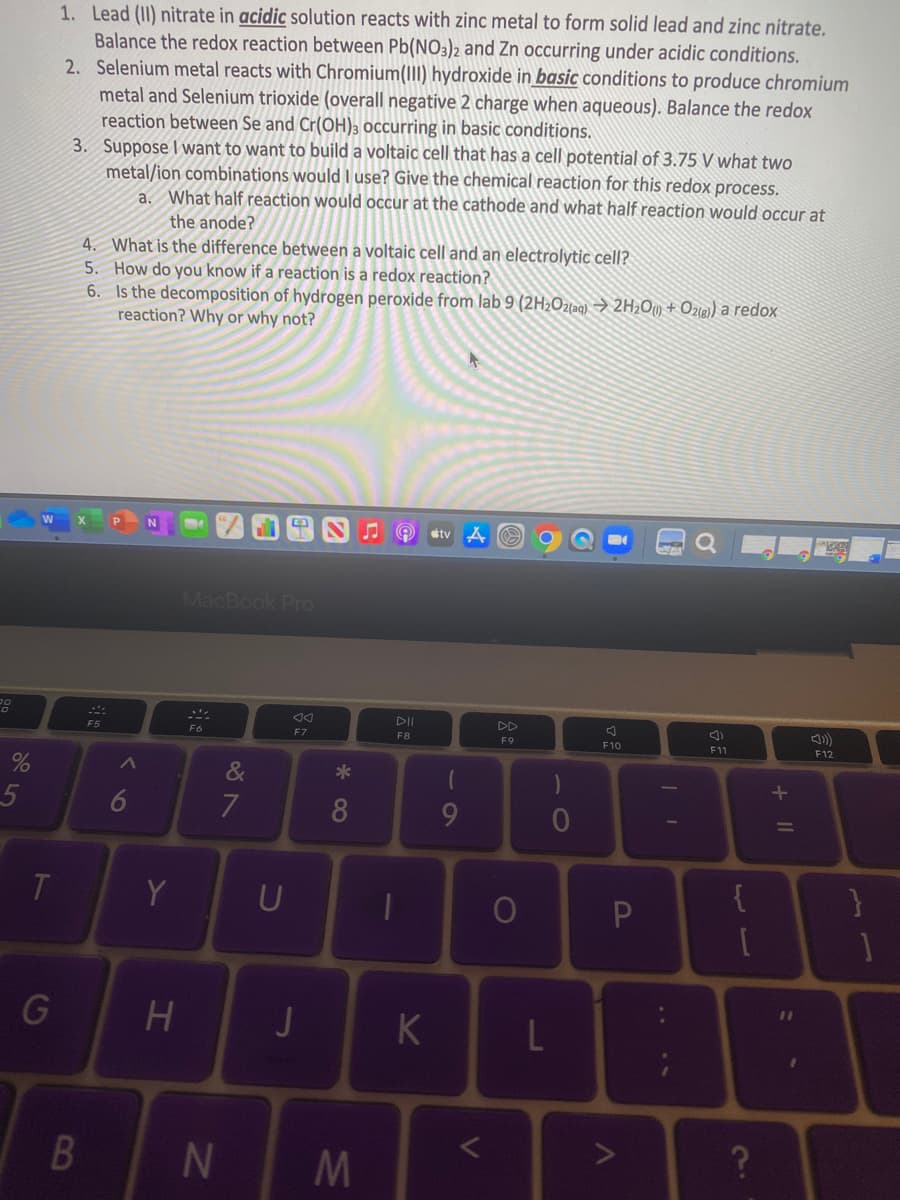
Transcribed Image Text:1. Lead (II) nitrate in acidic solution reacts with zinc metal to form solid lead and zinc nitrate.
Balance the redox reaction between Pb(NO3)2 and Zn occurring under acidic conditions.
2. Selenium metal reacts with Chromium(III) hydroxide in basic conditions to produce chromium
metal and Selenium trioxide (overall negative 2 charge when aqueous). Balance the redox
reaction between Se and Cr(OH)3 occurring in basic conditions.
3. Suppose I want to want to build a voltaic cell that has a cell potential of 3.75 V what two
metal/ion combinations would I use? Give the chemical reaction for this redox process.
a. What half reaction would occur at the cathode and what half reaction would occur at
the anode?
4. What is the difference between a voltaic cell and an electrolytic cell?
5. How do you know if a reaction is a redox reaction?
6. Is the decomposition of hydrogen peroxide from lab 9 (2H2Ozlag) → 2H2Oy + Ozie) a redox
reaction? Why or why not?
J O stv
MacBook Pro
F5
F6
F7
F8
F9
F10
F11
F12
&
*
9
T
Y
U
{
G
L
N
+ ||
....
* 00
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning