1. Nitrogen monoxide reacts with oxygen to form nitrogen dioxide. NO(g) + O2(g) → NO2(g) a. How many moles of 02 combine with 600 moles of NO? b. How many moles of NO2 are formed from 0.350 mole of NO and sufficient 02?
1. Nitrogen monoxide reacts with oxygen to form nitrogen dioxide. NO(g) + O2(g) → NO2(g) a. How many moles of 02 combine with 600 moles of NO? b. How many moles of NO2 are formed from 0.350 mole of NO and sufficient 02?
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 54QAP: Ammonia reacts with a limited amount of oxygen according to the equation...
Related questions
Question
100%
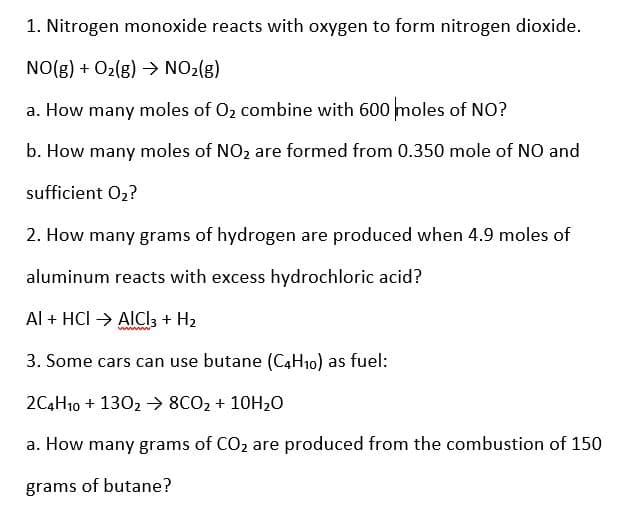
Transcribed Image Text:1. Nitrogen monoxide reacts with oxygen to form nitrogen dioxide.
NO(g) + O2(g) →> NO2(g)
a. How many moles of O2 combine with 600 moles of NO?
b. How many moles of NO2 are formed from 0.350 mole of NO and
sufficient 02?
2. How many grams of hydrogen are produced when 4.9 moles of
aluminum reacts with excess hydrochloric acid?
Al + HCI → AICI3 + H2
wwww
3. Some cars can use butane (C4H10) as fuel:
2C4H10 + 1302 → 8CO2 + 10H20
a. How many grams of CO, are produced from the combustion of 150
grams of butane?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning
