1. Relating Heat, Temperature Change, and Heat Capacity a. Large beds of rocks are used in some solar-heated homes to store heat. Assume that the specific heat of the rocks is 0.82 J/gK. Calculate the quantity of heat absorbed by 50.0 kg of rocks if their temperature increases by 12.0 °C. b. What temperature change would these rocks undergo if they emitted 450 kJ of heat?
1. Relating Heat, Temperature Change, and Heat Capacity a. Large beds of rocks are used in some solar-heated homes to store heat. Assume that the specific heat of the rocks is 0.82 J/gK. Calculate the quantity of heat absorbed by 50.0 kg of rocks if their temperature increases by 12.0 °C. b. What temperature change would these rocks undergo if they emitted 450 kJ of heat?
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter10: Energy
Section: Chapter Questions
Problem 68A
Related questions
Question
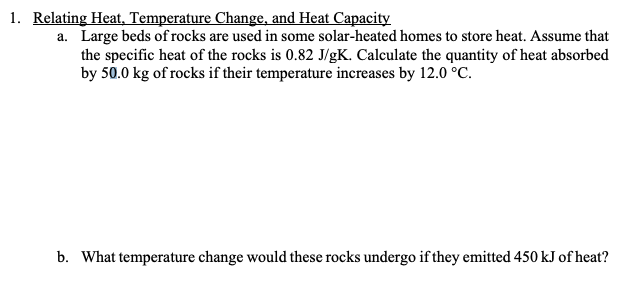
Transcribed Image Text:1. Relating Heat, Temperature Change, and Heat Capacity
a. Large beds of rocks are used in some solar-heated homes to store heat. Assume that
the specific heat of the rocks is 0.82 J/gK. Calculate the quantity of heat absorbed
by 50.0 kg of rocks if their temperature increases by 12.0 °C.
b. What temperature change would these rocks undergo if they emitted 450 kJ of heat?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning