1. Solve the following queries (parts a), b), & c)) dealing with volumetric analysis. Make sure you show complete procedures a) Ammonia reacts with hypobromite, OBr, by the reaction: 2NH3 + 30Br→ N₂ + 3Br + 3H₂O What is the molarity of a hypobromite solution if 1.00 mL of the OBr - solution reacts with 1.69 mg of NH3? b) A 0.8040-g sample of an iron ore is dissolved in acid. The iron is then reduced to Fe2+ and titrated with 47.22 mL of 0.02242 M KMnO4 solution. Calculate the results of this analysis in terms of (a) % Fe and (b) % Fe3O4. The reaction is the following one: MnO4 +5Fe²+ + 8H+ → Mn²+ +5Fe³+ + 4H₂O c) A solution of HCIO4 was standardized by dissolving 0.4008 g of primary-standard- grade HgO in a solution of KBr: HgO(s) + 4Br + H₂O → HgBr?¯ + 2OH- The liberated OH consumed 43.75 mL of the acid. Calculate the molar concentration of the HCIO4.
1. Solve the following queries (parts a), b), & c)) dealing with volumetric analysis. Make sure you show complete procedures a) Ammonia reacts with hypobromite, OBr, by the reaction: 2NH3 + 30Br→ N₂ + 3Br + 3H₂O What is the molarity of a hypobromite solution if 1.00 mL of the OBr - solution reacts with 1.69 mg of NH3? b) A 0.8040-g sample of an iron ore is dissolved in acid. The iron is then reduced to Fe2+ and titrated with 47.22 mL of 0.02242 M KMnO4 solution. Calculate the results of this analysis in terms of (a) % Fe and (b) % Fe3O4. The reaction is the following one: MnO4 +5Fe²+ + 8H+ → Mn²+ +5Fe³+ + 4H₂O c) A solution of HCIO4 was standardized by dissolving 0.4008 g of primary-standard- grade HgO in a solution of KBr: HgO(s) + 4Br + H₂O → HgBr?¯ + 2OH- The liberated OH consumed 43.75 mL of the acid. Calculate the molar concentration of the HCIO4.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 14.74QE: Lead poisoning has been a hazard for centuries. Some scholars believe that the decline of the Roman...
Related questions
Question
show full & complete procedure. Please answer parts a), b) & c). Note they are subparts of the same question
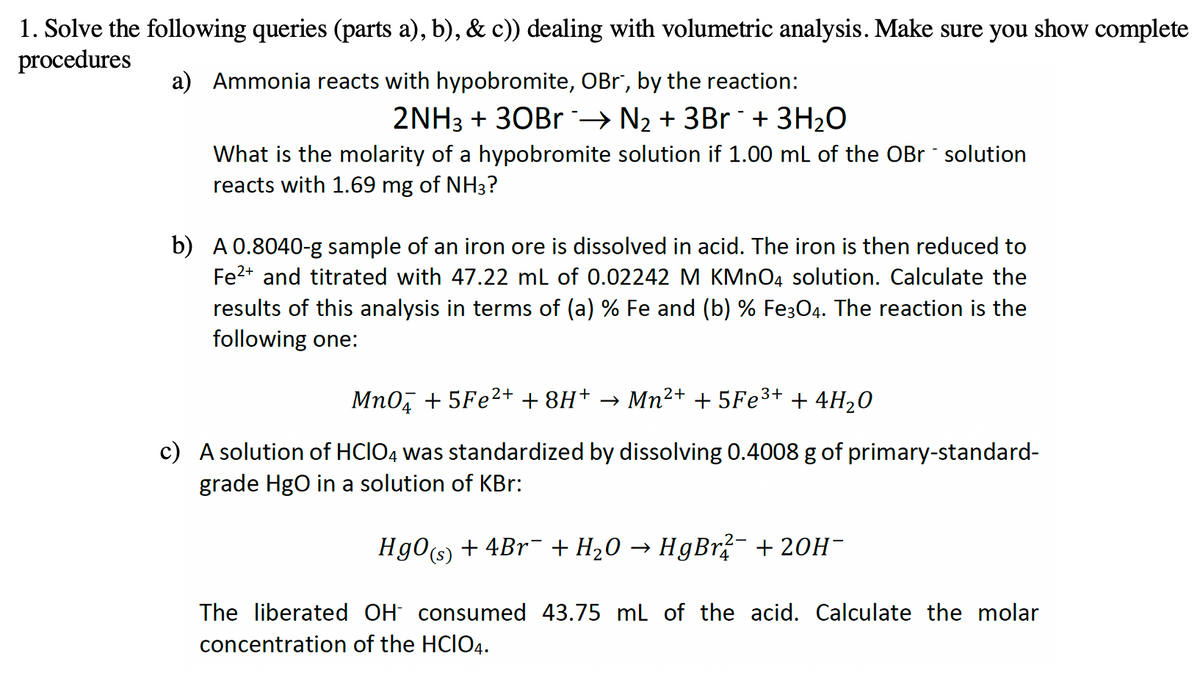
Transcribed Image Text:1. Solve the following queries (parts a), b), & c)) dealing with volumetric analysis. Make sure you show complete
procedures
a) Ammonia reacts with hypobromite, OBr", by the reaction:
2NH3 + 30Br ¯➜ N₂ + 3Br ¯ + 3H₂O
What is the molarity of a hypobromite solution if 1.00 mL of the OBr - solution
reacts with 1.69 mg of NH3?
b) A 0.8040-g sample of an iron ore is dissolved in acid. The iron is then reduced to
Fe²+ and titrated with 47.22 mL of 0.02242 M KMnO4 solution. Calculate the
results of this analysis in terms of (a) % Fe and (b) % Fe3O4. The reaction is the
following one:
MnO +5Fe²+ + 8H+ → Mn²+ + 5Fe³+ + 4H₂0
c) A solution of HCIO4 was standardized by dissolving 0.4008 g of primary-standard-
grade HgO in a solution of KBr:
HgO(s) + 4Br¯ + H₂O → HgBr²¯ +20H¯
The liberated OH consumed 43.75 mL of the acid. Calculate the molar
concentration of the HCIO4.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

