1. The following is an electron transfer reaction between two atoms: Ag + O ----> Ag2+ + O²- Which pair of sets of quantum numbers (n,l,mg) describe the initial and final orbitals of the SECOND transferred electron, respectively? H 2 Не 100 e 10 Li Be B o F Ne 11 12 13 14 15 16 17 18 Na Mg AI Si P ci Ar Markum 22 31 19 20 27 21 Sc Ti v Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 25 28 29 32 33 34 35 36 к Са 37 29 40 41 42 43 44 45 46 47 49 s0 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe AOAR 55 S7-70 71 72 73 74 75 76 77 78 79 82 14 Cs Ba Lu Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn 87 -102 103 104 105 109 110 111 114 106 107 108 112 Fr Ra ** Lr Rf Db Sg| Bh Hs Mt Uun Uuu Uub Uuq amaan "Lanthanide series 57 63 64 65 67 70 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb **Actinide series 92 95 101 93 14 96 97 99 100 102 Ac Th Pa ü Np Pu Am Cm Bk Cf Es Fm Md No 2 Select one: Oa. From (2,0, -1/2) to (5,1, +1/2) Ob. From (5,0, -1/2) to (2,1, -1/2) Oc. From (5,2,+1/2) to (2,1,+1/2) od. From (4,2, 1/2) to (2,1, 1/2) O e. From (2,1,-1/2) to (4,2,-1/2)
1. The following is an electron transfer reaction between two atoms: Ag + O ----> Ag2+ + O²- Which pair of sets of quantum numbers (n,l,mg) describe the initial and final orbitals of the SECOND transferred electron, respectively? H 2 Не 100 e 10 Li Be B o F Ne 11 12 13 14 15 16 17 18 Na Mg AI Si P ci Ar Markum 22 31 19 20 27 21 Sc Ti v Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 23 24 25 28 29 32 33 34 35 36 к Са 37 29 40 41 42 43 44 45 46 47 49 s0 53 54 Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe AOAR 55 S7-70 71 72 73 74 75 76 77 78 79 82 14 Cs Ba Lu Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn 87 -102 103 104 105 109 110 111 114 106 107 108 112 Fr Ra ** Lr Rf Db Sg| Bh Hs Mt Uun Uuu Uub Uuq amaan "Lanthanide series 57 63 64 65 67 70 La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb **Actinide series 92 95 101 93 14 96 97 99 100 102 Ac Th Pa ü Np Pu Am Cm Bk Cf Es Fm Md No 2 Select one: Oa. From (2,0, -1/2) to (5,1, +1/2) Ob. From (5,0, -1/2) to (2,1, -1/2) Oc. From (5,2,+1/2) to (2,1,+1/2) od. From (4,2, 1/2) to (2,1, 1/2) O e. From (2,1,-1/2) to (4,2,-1/2)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter6: Quantum Mechanics And Molecular Structure
Section: Chapter Questions
Problem 39P: The photoelectron spectrum of HBr has two main groups of peaks. The first has ionization energy...
Related questions
Question
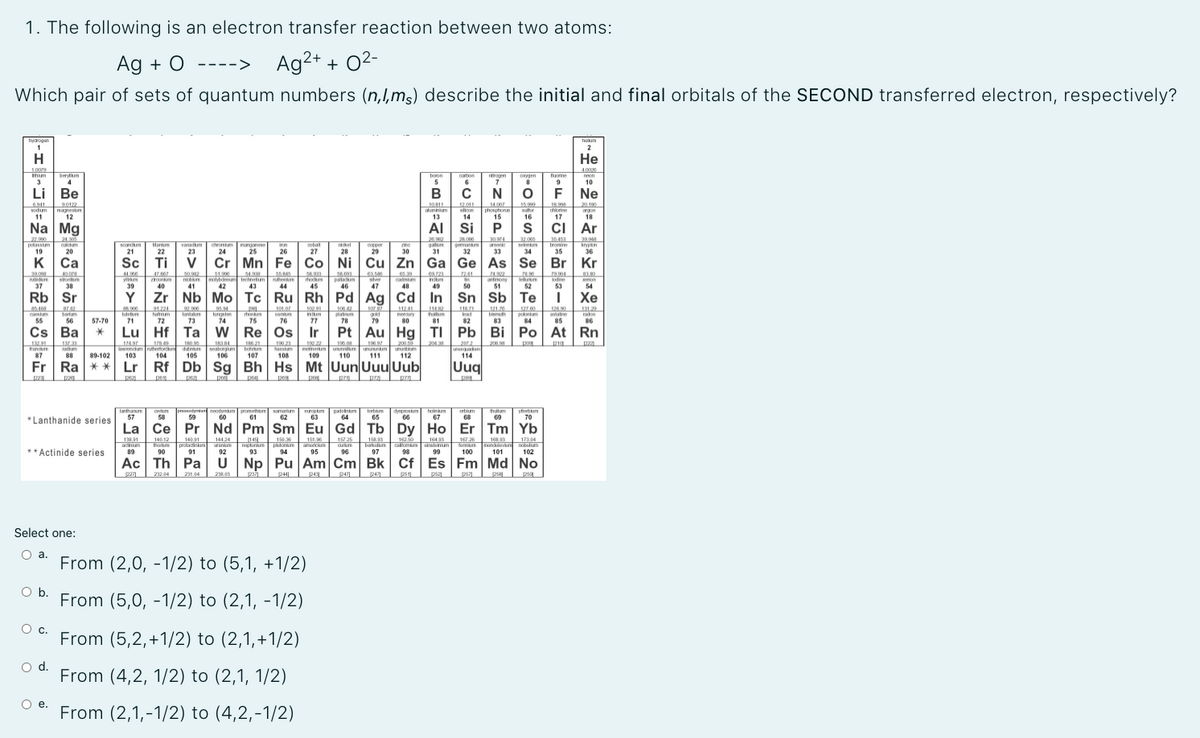
Transcribed Image Text:1. The following is an electron transfer reaction between two atoms:
Ag + O
Ag2+ + 02-
---->
Which pair of sets of quantum numbers (n,I,mg) describe the initial and final orbitals of the SECOND transferred electron, respectively?
hydrogan
1
haum
2
H
Не
1009
hum
4.0006
berytum
4
boon
carton
ORygen
fuorine
neon
3
5
6
10
Li Be
F Ne
18.M
chiorine
17
20.180
6941
sodum
90122
10811
alumm
12.011
sloon
15.999
sutur
magnestim
12
argon
11
13
14
16
18
Na Mg
AI Si
ci Ar
22.00
potassim
19
24.305
caicm
Hantm
22
chromium nganese
25
24
cobal
27
262
galum
31
26.0
gemanum
32
32.005
sekm
34
35453
tromine
Scondum
vanam
23
kel
krypn
36
ion
copper
20
21
26
20
29
30
35
к Са
Ca
Sc Ti
V
Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
nudum
37
40.A
Mrontum
38
44.
yum
39
roonm
40
1.000
iybden lectnetum
42
thenum
44
palam
46
65.30
camm
48
itodum
ser
dine
53
Indum
non
41
43
45
47
49
50
52
54
Rb Sr
Y
Zr Nb Mo Tc Ru Rh Pd Ag Cd In
Sn Sb Te
Хе
.000
etum
71
1.224
ham
or
114
halum
81
1200
casm
55
bartum
56
02.
laam
73
hingim
74
plaum
78
11241
meraury
80
12700
poknm
84
Inad
radon
86
Osum
astane
72
Lu Hf Ta w
57-70
75
76
77
79
82
85
Cs Ba
w Re Os Ir
Pt Au Hg TI
Pb Bi Po At Rn
12 91
180.95
ditum
105
1 22
memerum uunntum
109
2000
nut
112
2013
trandum
seaborgum
borm
hansm
108
ununm
87
88
89-102
103
104
106
107
110
111
114
Fr Ra
** Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub
Uuq
20
120
lantham
57
promethum smanm
61
uropu
hlm
69
cetum
prasendymtan eodmum
erekum
terbitum
holmum
ertium
yterum
58
65
*Lanthanide series
59
60
62
63
64
66
67
68
70
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb
138.91
16250
n 24 151. 151 162 50 167.28 188.
140.12
hom
90
140.91
prota m
91
144.24
149
150.36
putonum
94
157.25
158.93
164.93
168.93
mand n m
101
173.04
americum
bakum
97
Tamum
100
cuum
** Actinide series
89
92
93
95
96
98
99
102
Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No
pan
232.04
231.04
238.0s
pa
p4
psay
pse
psa
Select one:
O a.
From (2,0, -1/2) to (5,1, +1/2)
а.
Ob.
From (5,0, -1/2) to (2,1, -1/2)
c.
From (5,2,+1/2) to (2,1,+1/2)
d.
From (4,2, 1/2) to (2,1, 1/2)
Ое.
From (2,1,-1/2) to (4,2,-1/2)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER