1. The following is an electron transfer reaction between two atoms: + O ----> Ag2+ + O2- Which pair of sets of quantum numbers (n,l,m) describe the initial and final orbitals of the SECOND transferred electron, respectively? H Не 10 Li Be N F Ne 11 12 14 15 16 17 18 Na Mg AI Si S CI Ar 19 20 21 22 23 24 25 27 20 29 31 32 34 35 к Са Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 37 Rb Sr 40 41 42 43 44 45 46 47 49 52 53 54 Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe S7-70 71 72 73 14 75 76 77 78 79 12 84 Cs Ba Lu Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn 114 Uuq 102 103 104 105 100 108 109 110 111 112 Fr Ra * Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub 64 65 70 "Lanthanide series La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb *Actinide series 2 14 100 102 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No psa Select one: Oa. From (2,0, -1/2) to (5,1, +1/2) Ob. From (2,1,-1/2) to (4,2,-1/2) From (5,0, -1/2) to (2,1, -1/2) Od. From (5,2,+1/2) to (2,1,+1/2) Oe. O e. From (4,2, 1/2) to (2,1, 1/2)
1. The following is an electron transfer reaction between two atoms: + O ----> Ag2+ + O2- Which pair of sets of quantum numbers (n,l,m) describe the initial and final orbitals of the SECOND transferred electron, respectively? H Не 10 Li Be N F Ne 11 12 14 15 16 17 18 Na Mg AI Si S CI Ar 19 20 21 22 23 24 25 27 20 29 31 32 34 35 к Са Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 37 Rb Sr 40 41 42 43 44 45 46 47 49 52 53 54 Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe S7-70 71 72 73 14 75 76 77 78 79 12 84 Cs Ba Lu Hf Ta W Re Os Ir Pt Au Hg TI Pb Bi Po At Rn 114 Uuq 102 103 104 105 100 108 109 110 111 112 Fr Ra * Lr Rf Db Sg Bh Hs Mt Uun Uuu Uub 64 65 70 "Lanthanide series La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb *Actinide series 2 14 100 102 Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No psa Select one: Oa. From (2,0, -1/2) to (5,1, +1/2) Ob. From (2,1,-1/2) to (4,2,-1/2) From (5,0, -1/2) to (2,1, -1/2) Od. From (5,2,+1/2) to (2,1,+1/2) Oe. O e. From (4,2, 1/2) to (2,1, 1/2)
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter7: The Structure Of Atoms And Periodic Trends
Section: Chapter Questions
Problem 33PS: Identify the element that corresponds to each of the simplified photoelectron spectral data given...
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
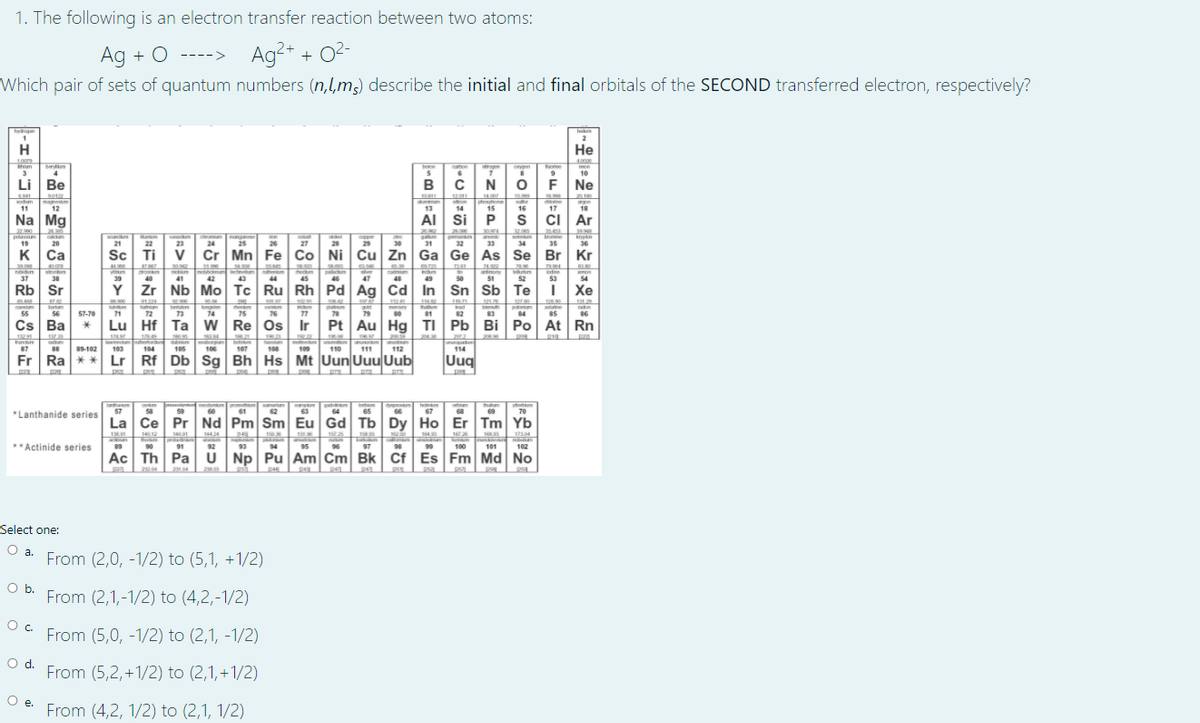
Transcribed Image Text:1. The following is an electron transfer reaction between two atoms:
Ag + O ----> Ag²+ + O2-
Which pair of sets of quantum numbers (n,l,m,) describe the initial and final orbitals of the SECOND transfered electron, respectively?
*Lanthanide series7
**Actinide series
Select one:
O a. From (2,0, -1/2) to (5,1, +1/2)
O b. From (2,1,-1/2) to (4,2,-1/2)
Oc From (5,0, -1/2) to (2,1, -1/2)
O d. From (5,2,+1/2) to (2,1, +1/2)
Pe. From (4,2, 1/2) to (2,1, 1/2)
に8Y”山5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning