Drinking of too much alcohol cause liver cirrhosis because ethanol is converted to toxic Acetone Ethanal Acetic acid O Methyl ethanoate Ethers have lower boiling points compared to alcohols of comparable mass because they form dipole-dipole interaction among themselves. they cannot form hydrogen bond among themselves. they can form hydrogen bond with water. they are solvents in organic reactions. Dehydration of two molecules of methanol under acidic condition (H2SO4) at high temperature will produce * Acetone Ethanal Acetic acid Methoxymethane O O O
Drinking of too much alcohol cause liver cirrhosis because ethanol is converted to toxic Acetone Ethanal Acetic acid O Methyl ethanoate Ethers have lower boiling points compared to alcohols of comparable mass because they form dipole-dipole interaction among themselves. they cannot form hydrogen bond among themselves. they can form hydrogen bond with water. they are solvents in organic reactions. Dehydration of two molecules of methanol under acidic condition (H2SO4) at high temperature will produce * Acetone Ethanal Acetic acid Methoxymethane O O O
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter13: Alcohols, Phenols, And Ethers
Section: Chapter Questions
Problem 13.69E: Figure 13.13 focuses on the use of thiol chemistry by skunks. If thiols are more volatile than...
Related questions
Question
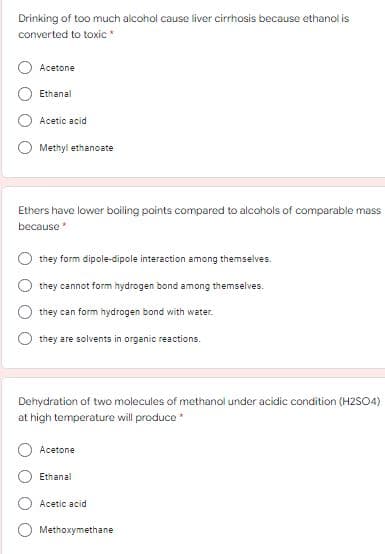
Transcribed Image Text:Drinking of too much alcohol cause liver cirrhosis because ethanol is
converted to toxic *
Acetone
Ethanal
Acetic acid
Methyl ethanoate
Ethers have lower boiling points compared to alcohols of comparable mass
because
they form dipole-dipole interaction among themselves.
they cannot form hydrogen bond among themselves.
they can form hydrogen bond with water.
they are solvents in organic reactions.
Dehydration of two molecules of methanol under acidic condition (H2SO4)
at high temperature will produce *
Acetone
Ethanal
Acetic acid
Methoxymethane
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning