1. The region of electromagnetic spectrum for nuclear magnetic resonance is: a) Microwave b) UV-rays c) Infrared 2. The wave numbers of the radiations with a wavelength a) 0 43 x 105 Radio frequency
1. The region of electromagnetic spectrum for nuclear magnetic resonance is: a) Microwave b) UV-rays c) Infrared 2. The wave numbers of the radiations with a wavelength a) 0 43 x 105 Radio frequency
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 39P: Chapter 3 introduced the concept of a double bond between carbon atoms, represented by C=C , with a...
Related questions
Question
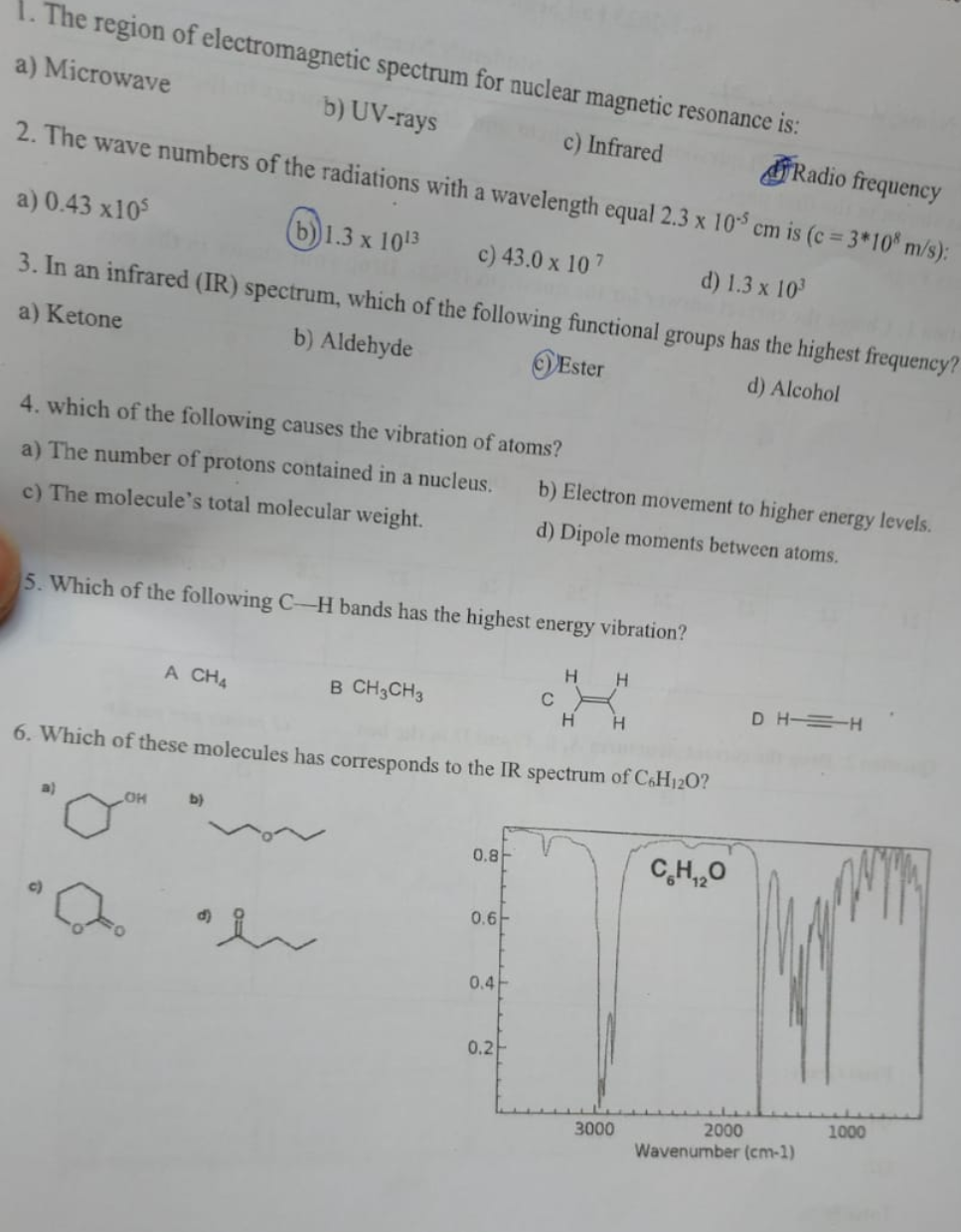
Transcribed Image Text:1. The region of electromagnetic spectrum for nuclear magnetic resonance is:
a) Microwave
b) UV-rays
c) Infrared
Radio frequency
2. The wave numbers of the radiations with a wavelength equal 2.3 x 105 cm is (c = 3*108 m/s):
a) 0.43 x105
b) 1.3 x 1013
c) 43.0 x 107
d) 1.3 x 10³
3. In an infrared (IR) spectrum, which of the following functional groups has the highest frequency?
a) Ketone
b) Aldehyde
Ester
d) Alcohol
4. which of the following causes the vibration of atoms?
a) The number of protons contained in a nucleus.
c) The molecule's total molecular weight.
5. Which of the following C-H bands has the highest energy vibration?
a)
A CH4
LOH
b)
B CH3CH3
a h
6. Which of these molecules has corresponds to the IR spectrum of C6H120?
0.8
0.6
b) Electron movement to higher energy levels.
d) Dipole moments between atoms.
0.4
0.2
C
H
H
H H
3000
CH,,O
DHH
2000
Wavenumber (cm-1)
1000
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
