1. The solubility of magnesium hydroxide (Mg(OH)2) is approximately 3.70 x10-³ M at room temperature. a. Write the aqueous dissolution ionic reaction for magnesium hydroxide. Include state symbols. b. Calculate the solubility product constant (Ksp) for magnesium hydroxide at room temperature. Refer back to the General Formula for “K" presented in the "Equilibrium Lab Manual" c. How many grams of magnesium hydroxide are present in 1.0 L of a saturated solution at room temperature?
1. The solubility of magnesium hydroxide (Mg(OH)2) is approximately 3.70 x10-³ M at room temperature. a. Write the aqueous dissolution ionic reaction for magnesium hydroxide. Include state symbols. b. Calculate the solubility product constant (Ksp) for magnesium hydroxide at room temperature. Refer back to the General Formula for “K" presented in the "Equilibrium Lab Manual" c. How many grams of magnesium hydroxide are present in 1.0 L of a saturated solution at room temperature?
Chapter10: Reconstitution Of Powdered Drugs
Section: Chapter Questions
Problem 42SST
Related questions
Question
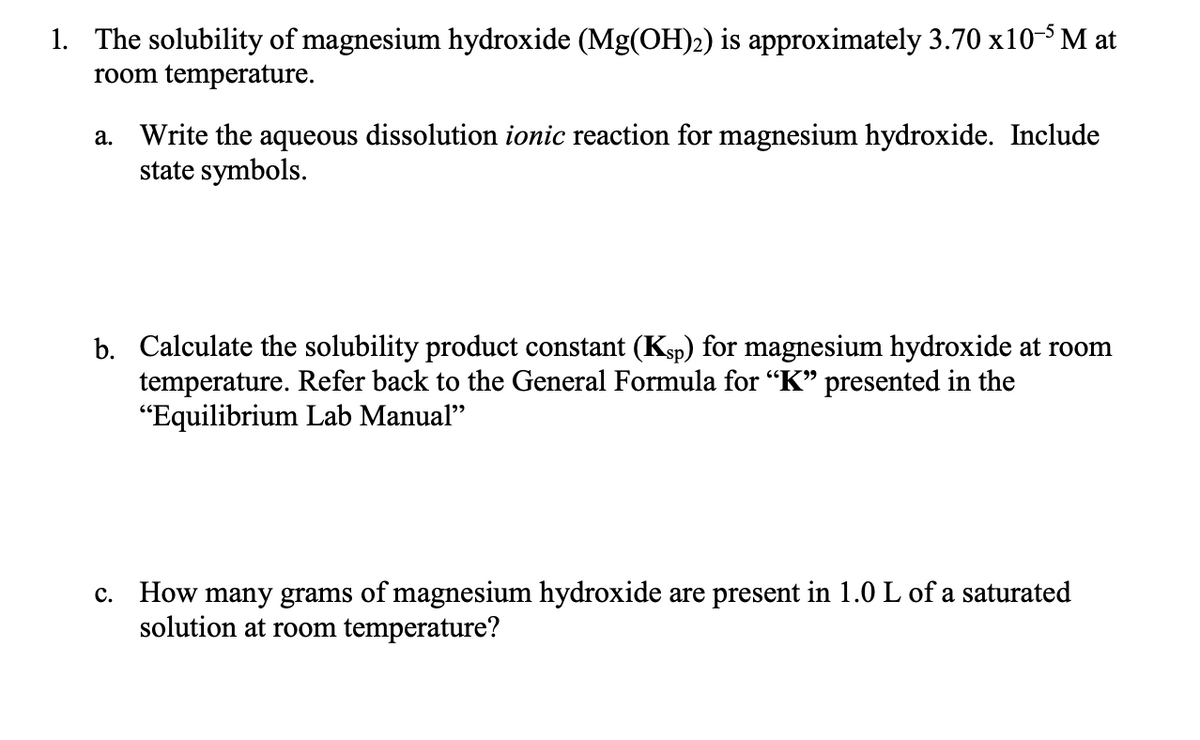
Transcribed Image Text:1. The solubility of magnesium hydroxide (Mg(OH)2) is approximately 3.70 x10- M at
room temperature.
a. Write the aqueous dissolution ionic reaction for magnesium hydroxide. Include
state symbols.
b. Calculate the solubility product constant (Ksp) for magnesium hydroxide at room
temperature. Refer back to the General Formula for “K" presented in the
"Equilibrium Lab Manual"
c. How many grams of magnesium hydroxide are present in 1.0 L of a saturated
solution at room temperature?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

