As a technician in a large pharmaceutical research firm, you need to produce 200. mL of 1.00 M potassium phosphate buffer solution of pH = 6.96. The pKa of H2 PO4 is 7.21. %3D You have the following supplies: 2.00 L of 1.00 M KH2PO4 stock solution, 1.50 L of 1.00 M K2 HPO4 stock solution, and a carboy of pure distilled H20. How much 1.00 M KH2PO4 will you need to make this solution? Express your answer to three significant digits with the appropriate units. > View Available Hint(s) HA ? Volume of KH2PO4 needed = Valıge Units
As a technician in a large pharmaceutical research firm, you need to produce 200. mL of 1.00 M potassium phosphate buffer solution of pH = 6.96. The pKa of H2 PO4 is 7.21. %3D You have the following supplies: 2.00 L of 1.00 M KH2PO4 stock solution, 1.50 L of 1.00 M K2 HPO4 stock solution, and a carboy of pure distilled H20. How much 1.00 M KH2PO4 will you need to make this solution? Express your answer to three significant digits with the appropriate units. > View Available Hint(s) HA ? Volume of KH2PO4 needed = Valıge Units
Chapter10: Reconstitution Of Powdered Drugs
Section: Chapter Questions
Problem 42SST
Related questions
Question
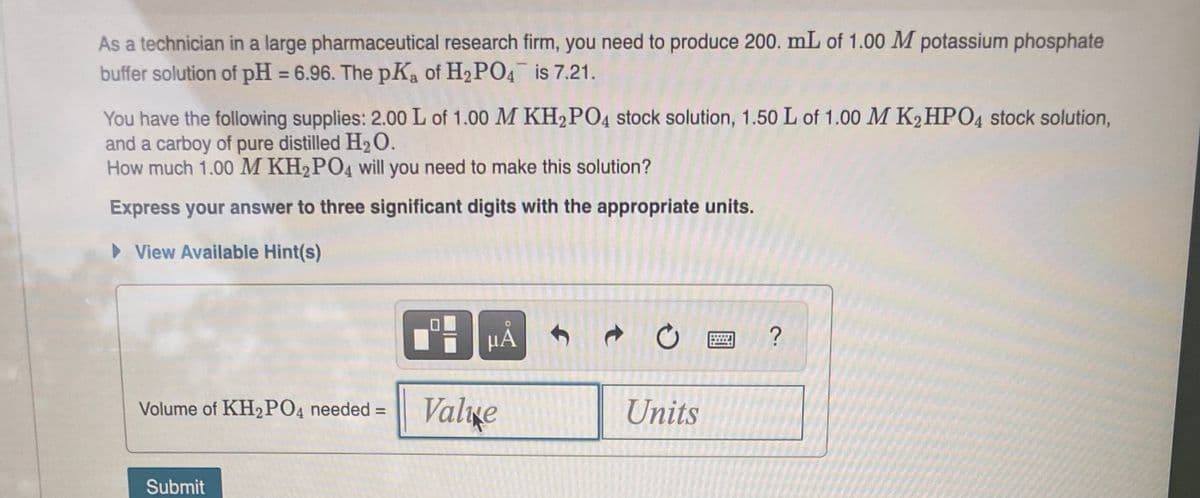
Transcribed Image Text:As a technician in a large pharmaceutical research firm, you need to produce 200. mL of 1.00 M potassium phosphate
buffer solution of pH = 6.96. The pKa of H2 PO4 is 7.21.
%3D
You have the following supplies: 2.00 L of 1.00 M KH2PO4 stock solution, 1.50 L of 1.00 M K2HPO4 stock solution,
and a carboy of pure distilled H2O.
How much 1.00 M KH2PO4 will you need to make this solution?
Express your answer to three significant digits with the appropriate units.
> View Available Hint(s)
HẢ
Volume of KH2PO4 needed =
Valıxe
Units
Submit
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



