1. Use the following data for NH3 (g) at T= 273 K to evaluate B2p(T). (Here Z is the compressibility factor, PV/RT). P (bar) Z-1 0.10 1.519 x 10-4 0.20 3.038 x 10-4 0.30 4.557x 104 0.40 6.071 x 10-4 0.50 7.583 x 104 0.60 9.002 x 10-4 0.70 1.0551 x 10-3 Hint: You will need to fit this data to a least-squares line, which can be done most easily in either Excel or in WolframAlpha.
1. Use the following data for NH3 (g) at T= 273 K to evaluate B2p(T). (Here Z is the compressibility factor, PV/RT). P (bar) Z-1 0.10 1.519 x 10-4 0.20 3.038 x 10-4 0.30 4.557x 104 0.40 6.071 x 10-4 0.50 7.583 x 104 0.60 9.002 x 10-4 0.70 1.0551 x 10-3 Hint: You will need to fit this data to a least-squares line, which can be done most easily in either Excel or in WolframAlpha.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 81AP
Related questions
Question
question 1 plese,little help..
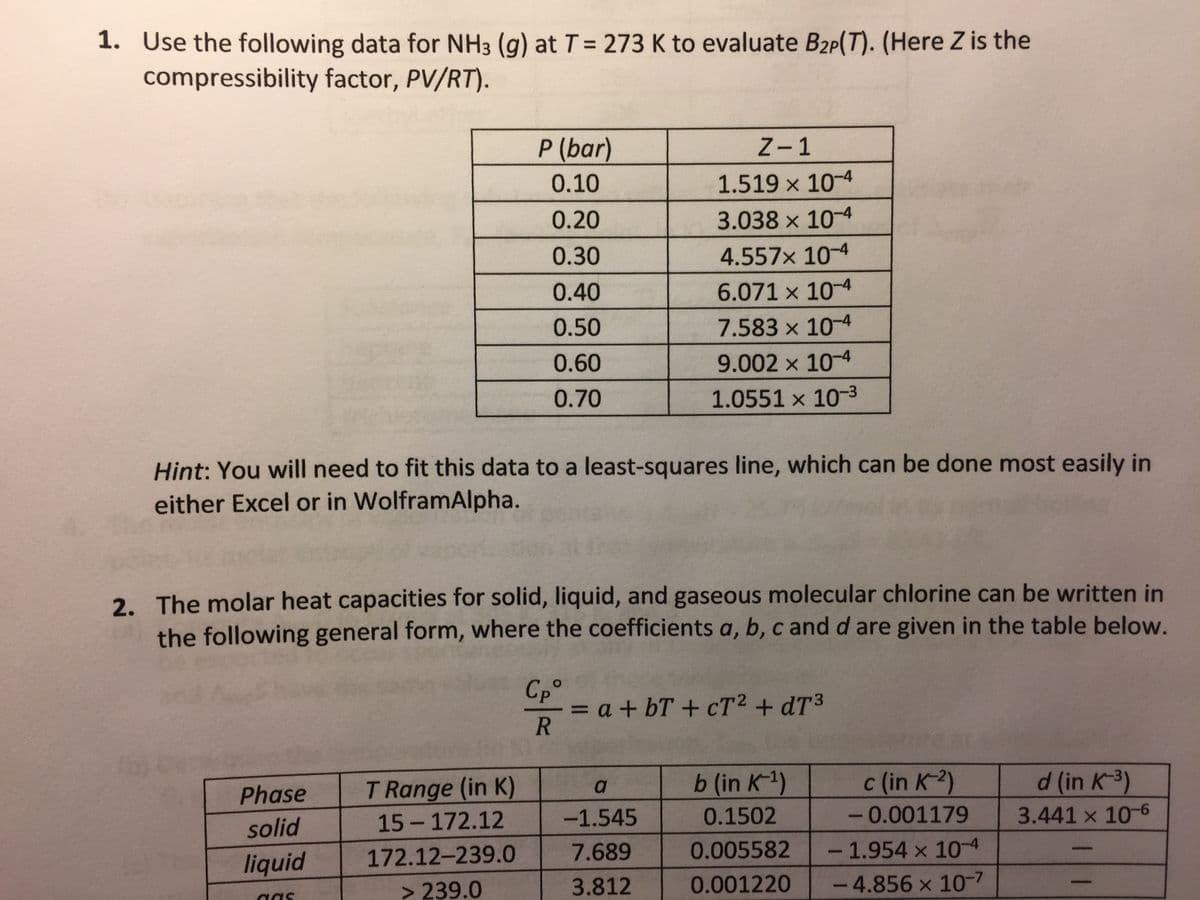
Transcribed Image Text:1. Use the following data for NH3 (g) at T= 273 K to evaluate B2p(T). (Here Z is the
compressibility factor, PV/RT).
P (bar)
Z-1
0.10
1.519 x 10-4
0.20
3.038 x 10-4
0.30
4.557x 10-4
0.40
6.071 x 10-4
0.50
7.583 x 10-4
0.60
9.002 x 10-4
0.70
1.0551 x 10-3
Hint: You will need to fit this data to a least-squares line, which can be done most easily in
either Excel or in WolframAlpha.
2. The molar heat capacities for solid, liquid, and gaseous molecular chlorine can be written in
the following general form, where the coefficients a, b, c and d are given in the table below.
Cp°
= a + bT + cT2 + dT3
T Range (in K)
b (in K-1)
c (in K-2)
d (in K-3)
a
Phase
15 - 172.12
-1.545
0.1502
-0.001179
3.441 x 10-6
solid
172.12-239.0
7.689
0.005582
- 1.954 x 104
liquid
> 239.0
3.812
0.001220
-4.856 x 10-7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning