What is the final temeprautre of a monoatomic ideal gas that is compressed reversible and adiabatically from 16 L to 2L at 300 K? O 1200 K 2400 K 1045 K 600 K
What is the final temeprautre of a monoatomic ideal gas that is compressed reversible and adiabatically from 16 L to 2L at 300 K? O 1200 K 2400 K 1045 K 600 K
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 72QAP: On complete combustion at constant pressure, a 1.00-L sample of a gaseous mixture at 0C and 1.00 atm...
Related questions
Question
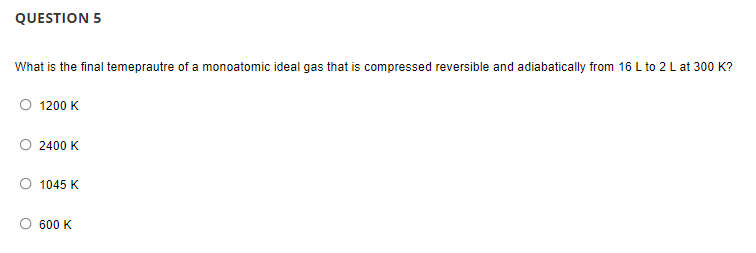
Transcribed Image Text:QUESTION 5
What is the final temeprautre of a monoatomic ideal gas that is compressed reversible and adiabatically from 16 L to 2 L at 300 K?
1200 K
2400 K
1045 K
600 K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning