1. Using the enthalpy of reaction he data at 3000K and the Kp value at 2800 K of the combustion process A2 + B₂ = 2AB (Kp = 0.385 @2800 K) a) Estimate the Kp value at 3200 K ) Determine the equilibrium mole fraction of AB when the pressure is 101 kPa and the temperature is 3200 K h₂98k h 3000K kJ/kmol kJ/kmol A2 B2 AB hi kJ/kmol 0 0 91,290 8,682 8,669 0 106,780 105,115 94,970
1. Using the enthalpy of reaction he data at 3000K and the Kp value at 2800 K of the combustion process A2 + B₂ = 2AB (Kp = 0.385 @2800 K) a) Estimate the Kp value at 3200 K ) Determine the equilibrium mole fraction of AB when the pressure is 101 kPa and the temperature is 3200 K h₂98k h 3000K kJ/kmol kJ/kmol A2 B2 AB hi kJ/kmol 0 0 91,290 8,682 8,669 0 106,780 105,115 94,970
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
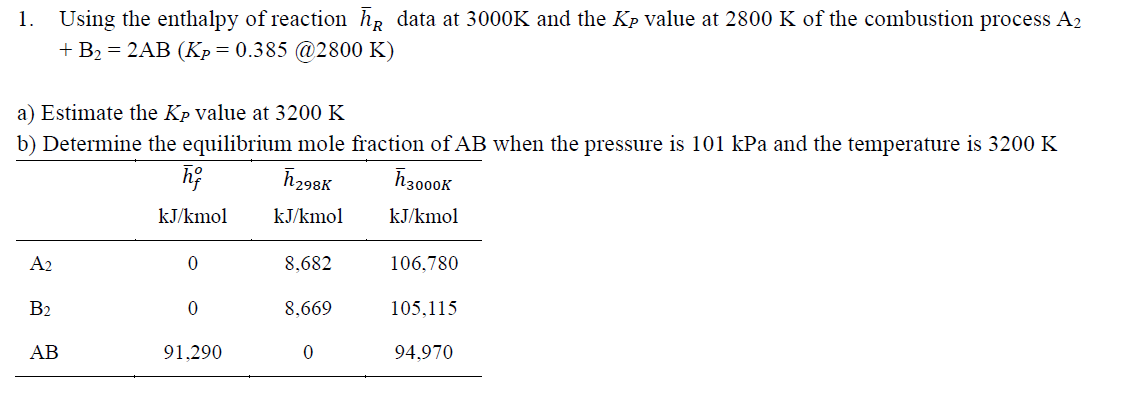
Transcribed Image Text:Using the enthalpy of reaction he data at 3000K and the Kp value at 2800 K of the combustion process A2
+ B2 = 2AB (Kp = 0.385 @2800 K)
1.
a) Estimate the Kp value at 3200 K
b) Determine the equilibrium mole fraction of AB when the pressure is 101 kPa and the temperature is 3200 K
ħ298K
ħ3000K
kJ/kmol
kJ/kmol
kJ/kmol
A2
8,682
106,780
B2
8,669
105,115
АВ
91,290
94,970
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY