Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 36QAP
Related questions
Question
Transcribed Image Text:3/a/NDQ3NTA1 NDIOMDQ2/details
int High Sc.
SIS Grades and Attend..
G Home Schoology
Ed puzzle
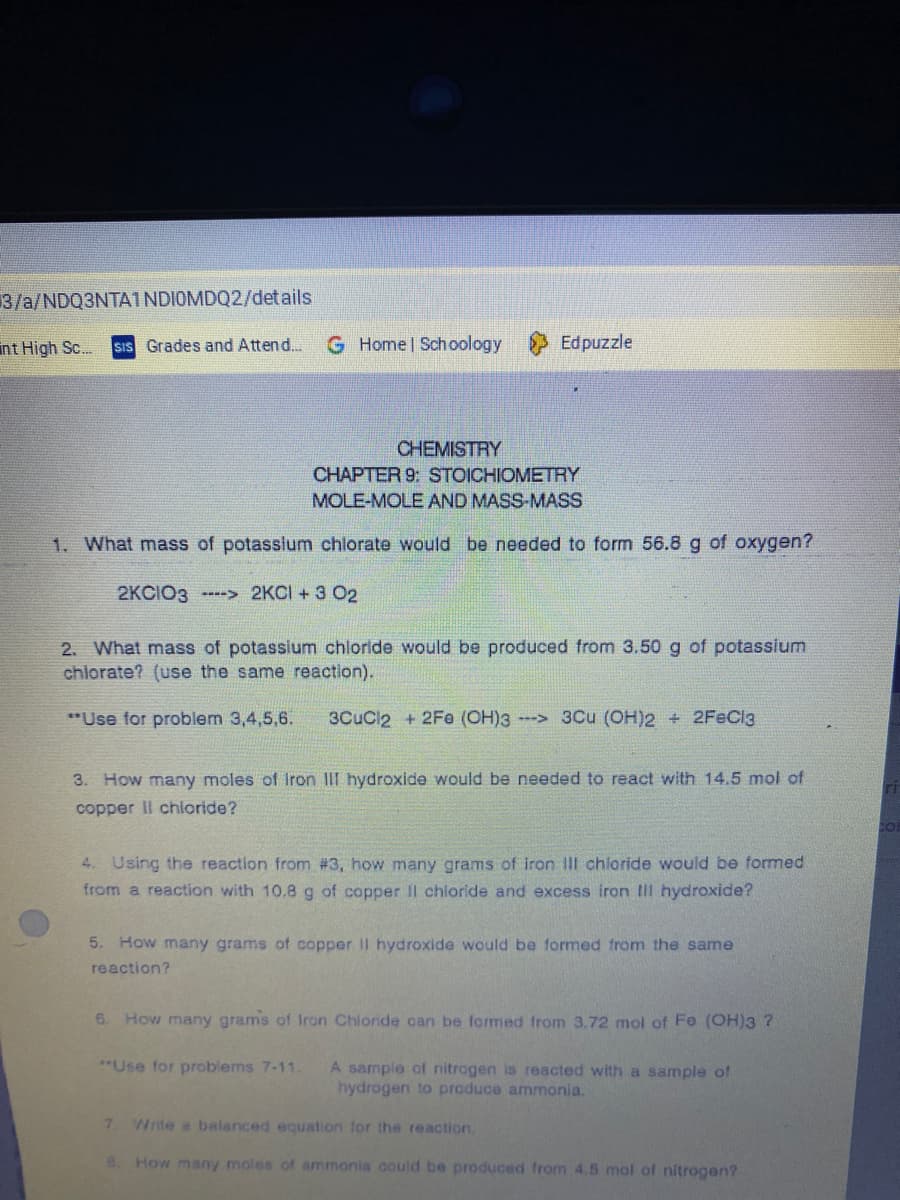
CHEMISTRY
CHAPTER 9: STOICHIOMETRY
MOLE-MOLE AND MASS-MASS
1. What mass of potasslum chlorate would be needed to form 56.8 g of oxygen?
2KCIO3 ----> 2KCI + 3 O2
2. What mass of potassium chloride would be produced from 3.50 g of potassium
chiorate? (use the same reaction).
"Use for problem 3,4,5,6.
3CuCl2 + 2Fe (OH)3-> 3Cu (OH)2 + 2FeCl3
3. How many moles of Iron III hydroxide would be needed to react with 14.5 mol of
copper II chioride?
4. Using the reaction from #3, how many grams of iron IIl chloride would be formed
from a reaction with 10.8 g of copper Il chloride and excess iron Ill hydroxide?
5. How many grams of copper II hydroxide would be formed from the same
reaction?
6. How many grams of Iron Chloride can be formed from 3.72 mol of Fe (OH)3?
*"Use for problems 7-11.
A sample of nitrogen is reacted with a sample of
hydrogen to produce ammonia.
7.
Write a balanced equation for the reaction.
8. How many moles of ammonia could be produced from 4.5 mol of nitrogen?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning