1. Which of the following statement is wrong: Specific heat of a material a. Extrinsic property b. Has unit of J/kg-C c. Constant for a material d. Heat capacity per unit mass 2. Which of the following cannot leave or enter a closed system? a. heat b. work 3. What is 4.184 J? c. matter a. The heat required to raise the temperature of one gram of water by 1°C. b. The heat required to raise the temperature of one mole of water by 1°C. c. The heat required to raise the temperature of one gram of substance by 1°C. d. The heat required to raise the temperature of one mole of substance by 1°C. 4. This is the amount of heat required to raise the temperature of an object by 1 K (or 1 °C). a. heat capacity b. specific heat capacity c. molar heat capacity d. enthalpy 5. Which of the following is transferred due to temperature change? a. Chemical energy d. energy b. mechanical energy c. electrical energy d. heat
1. Which of the following statement is wrong: Specific heat of a material a. Extrinsic property b. Has unit of J/kg-C c. Constant for a material d. Heat capacity per unit mass 2. Which of the following cannot leave or enter a closed system? a. heat b. work 3. What is 4.184 J? c. matter a. The heat required to raise the temperature of one gram of water by 1°C. b. The heat required to raise the temperature of one mole of water by 1°C. c. The heat required to raise the temperature of one gram of substance by 1°C. d. The heat required to raise the temperature of one mole of substance by 1°C. 4. This is the amount of heat required to raise the temperature of an object by 1 K (or 1 °C). a. heat capacity b. specific heat capacity c. molar heat capacity d. enthalpy 5. Which of the following is transferred due to temperature change? a. Chemical energy d. energy b. mechanical energy c. electrical energy d. heat
Physics for Scientists and Engineers, Technology Update (No access codes included)
9th Edition
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter20: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 20.3OQ: Assume you are measuring the specific heat of a sample of originally hot metal by using a...
Related questions
Question
Topic: General Physics
can you answer this 5 multiple choice question for my assignment please?
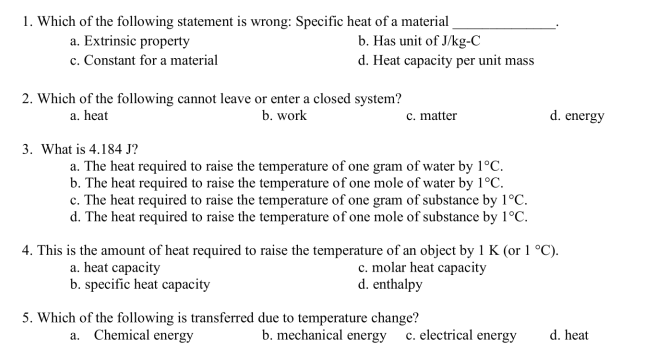
Transcribed Image Text:1. Which of the following statement is wrong: Specific heat of a material
a. Extrinsic property
b. Has unit of J/kg-C
c. Constant for a material
d. Heat capacity per unit mass
2. Which of the following cannot leave or enter a closed system?
a. heat
b. work
3. What is 4.184 J?
c. matter
a. The heat required to raise the temperature of one gram of water by 1°C.
b. The heat required to raise the temperature of one mole of water by 1°C.
c. The heat required to raise the temperature of one gram of substance by 1°C.
d. The heat required to raise the temperature of one mole of substance by 1°C.
4. This is the amount of heat required to raise the temperature of an object by 1 K (or 1 °C).
a. heat capacity
b. specific heat capacity
c. molar heat capacity
d. enthalpy
5. Which of the following is transferred due to temperature change?
a. Chemical energy
d. energy
b. mechanical energy c. electrical energy
d. heat
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Physics for Scientists and Engineers with Modern …
Physics
ISBN:
9781337553292
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning