1. Write a balanced chemical equation, including physical state symbols, for the decomposition of liquid nitroglycerin (C3H3 (NO;),) into gaseous dinitrogen, gaseous dioxygen, gaseous water and gaseous carbon dioxide. 2. Suppose 12.0 L of carbon dioxide gas are produced by this reaction, at a temperature of - 5.0 °C and pressure of exactly 1 atm. Calculate the mass of nitroglycerin that must have reacted. Be sure your answer has the correct number of significant digits.
1. Write a balanced chemical equation, including physical state symbols, for the decomposition of liquid nitroglycerin (C3H3 (NO;),) into gaseous dinitrogen, gaseous dioxygen, gaseous water and gaseous carbon dioxide. 2. Suppose 12.0 L of carbon dioxide gas are produced by this reaction, at a temperature of - 5.0 °C and pressure of exactly 1 atm. Calculate the mass of nitroglycerin that must have reacted. Be sure your answer has the correct number of significant digits.
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.76P
Related questions
Question
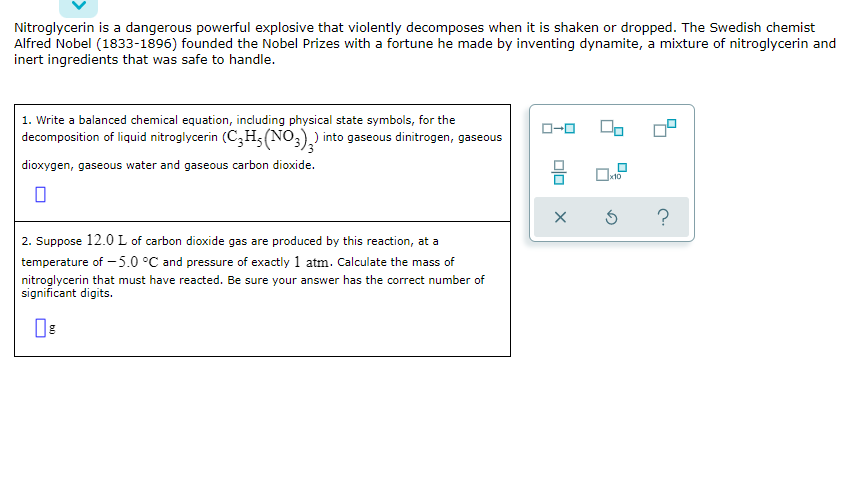
Transcribed Image Text:Nitroglycerin is a dangerous powerful explosive that violently decomposes when it is shaken or dropped. The Swedish chemist
Alfred Nobel (1833-1896) founded the Nobel Prizes with a fortune he made by inventing dynamite, a mixture of nitroglycerin and
inert ingredients that was safe to handle.
1. Write a balanced chemical equation, including physical state symbols, for the
decomposition of liquid nitroglycerin (C,H5(NO,),) into gaseous dinitrogen, gaseous
O-0
dioxygen, gaseous water and gaseous carbon dioxide.
2. Suppose 12.0 L of carbon dioxide gas are produced by this reaction, at a
temperature of – 5.0 °C and pressure of exactly 1 atm. Calculate the mass of
nitroglycerin that must have reacted. Be sure your answer has the correct number of
significant digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning