1. Write the balanced formula, complete ionic, and net ionic equations for the precipitation reaction between aluminum nitrate and potassium hydroxide, including all phase symbols. 2. Write the balanced formula, complete ionic, and net ionic equations for the reaction between the precipitate from question one and acetic acid, including all phase symbols. 3. From the reaction matrices below, identify the unknown compounds A, B, C, and D. known NazSO4 BaCl2 КОН Al(NO:); unknown A C D dense ppt, insoluble ppt, insoluble ppt, soluble Na,SO, no rxn no ixn no rxn dense ppt, insoluble dense ppt, soluble gel ppt, soluble BaCl2 no rxn B no rxn no rxn dense ppt, soluble gel ppt, soluble dense ppt, insoluble КОН no xn X C no rxn X no rxn gel ppt, soluble dense ppt, soluble gel ppt, soluble Al(NO;)3 no xn X D no rxn no rxn X
1. Write the balanced formula, complete ionic, and net ionic equations for the precipitation reaction between aluminum nitrate and potassium hydroxide, including all phase symbols. 2. Write the balanced formula, complete ionic, and net ionic equations for the reaction between the precipitate from question one and acetic acid, including all phase symbols. 3. From the reaction matrices below, identify the unknown compounds A, B, C, and D. known NazSO4 BaCl2 КОН Al(NO:); unknown A C D dense ppt, insoluble ppt, insoluble ppt, soluble Na,SO, no rxn no ixn no rxn dense ppt, insoluble dense ppt, soluble gel ppt, soluble BaCl2 no rxn B no rxn no rxn dense ppt, soluble gel ppt, soluble dense ppt, insoluble КОН no xn X C no rxn X no rxn gel ppt, soluble dense ppt, soluble gel ppt, soluble Al(NO;)3 no xn X D no rxn no rxn X
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 68QAP: 68. Aluminum ion may be precipitated from aqueous solution by addition of hydroxide ion, forming...
Related questions
Question
100%
Please answer all parts to questions 1 through 3.
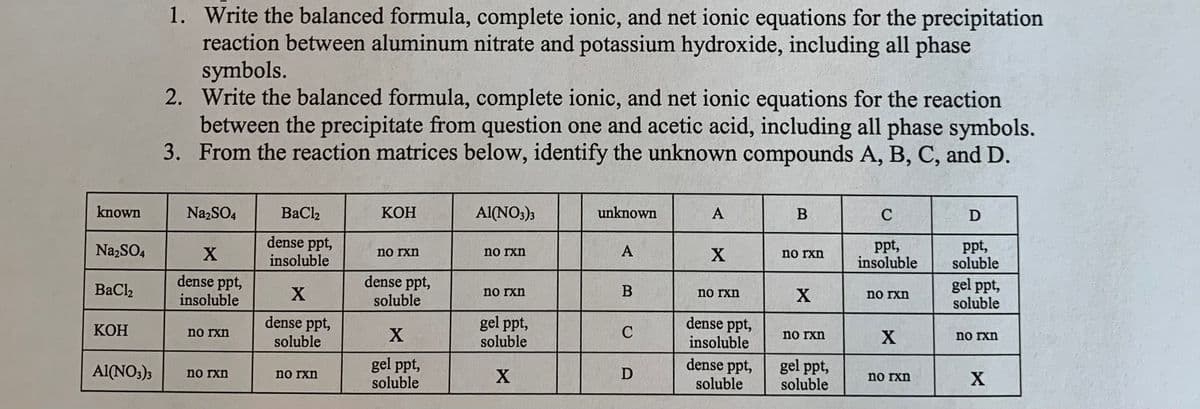
Transcribed Image Text:1. Write the balanced formula, complete ionic, and net ionic equations for the precipitation
reaction between aluminum nitrate and potassium hydroxide, including all phase
symbols.
2. Write the balanced formula, complete ionic, and net ionic equations for the reaction
between the precipitate from question one and acetic acid, including all phase symbols.
3. From the reaction matrices below, identify the unknown compounds A, B, C, and D.
known
NazSO4
BaCl2
КОН
Al(NO;)3
unknown
A
C
D
dense ppt,
ppt,
insoluble
ppt,
soluble
Na,SO4
no rxn
no rxn
A
X
no rxn
insoluble
dense ppt,
insoluble
dense ppt,
soluble
gel ppt,
soluble
BaCl,
no rxn
B
no rxn
no rxn
dense ppt,
gel ppt,
soluble
dense ppt,
insoluble
КОН
no rxn
no rxn
no rxn
soluble
dense ppt,
gel ppt,
soluble
gel ppt,
soluble
Al(NO:);
no rxn
no rxn
D
no rxn
soluble
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning
